Abstract
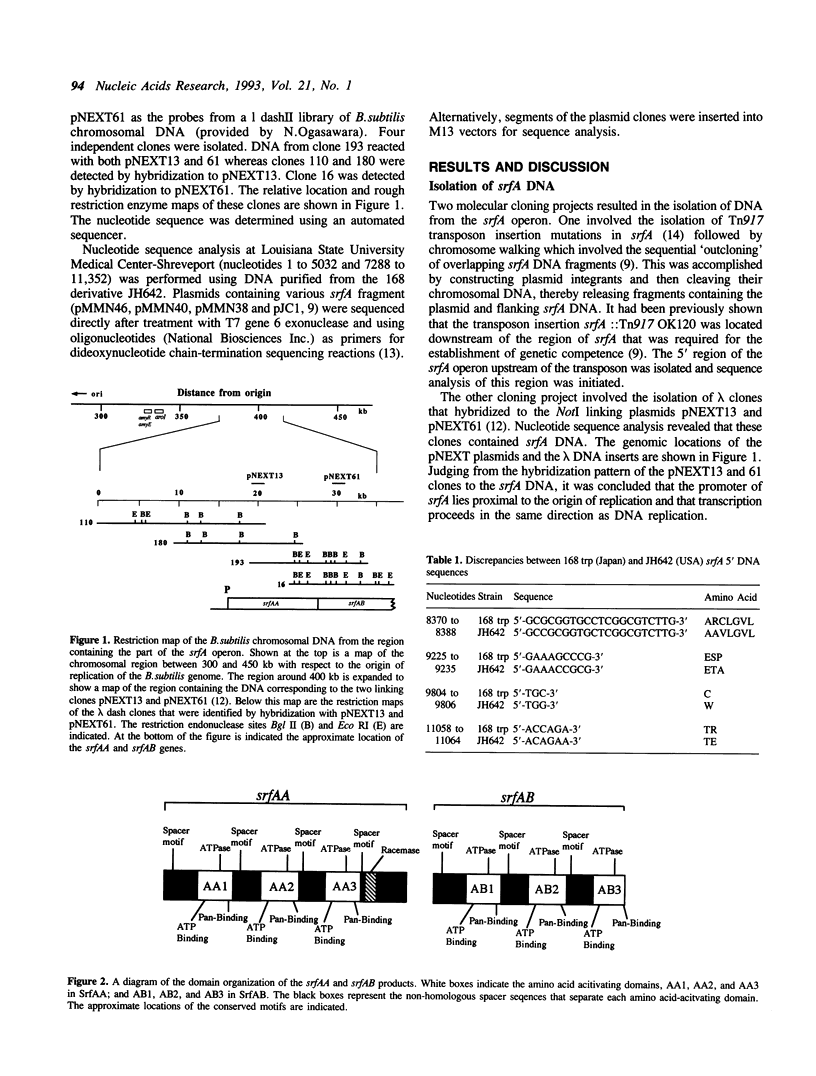
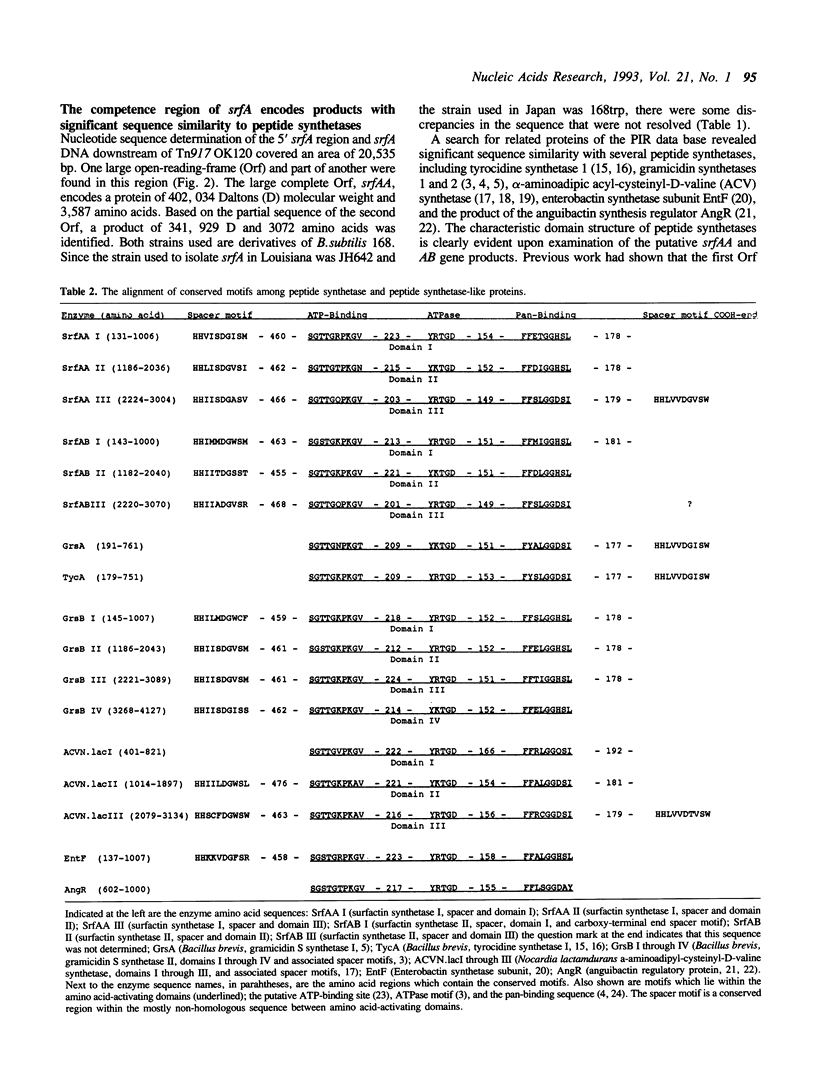
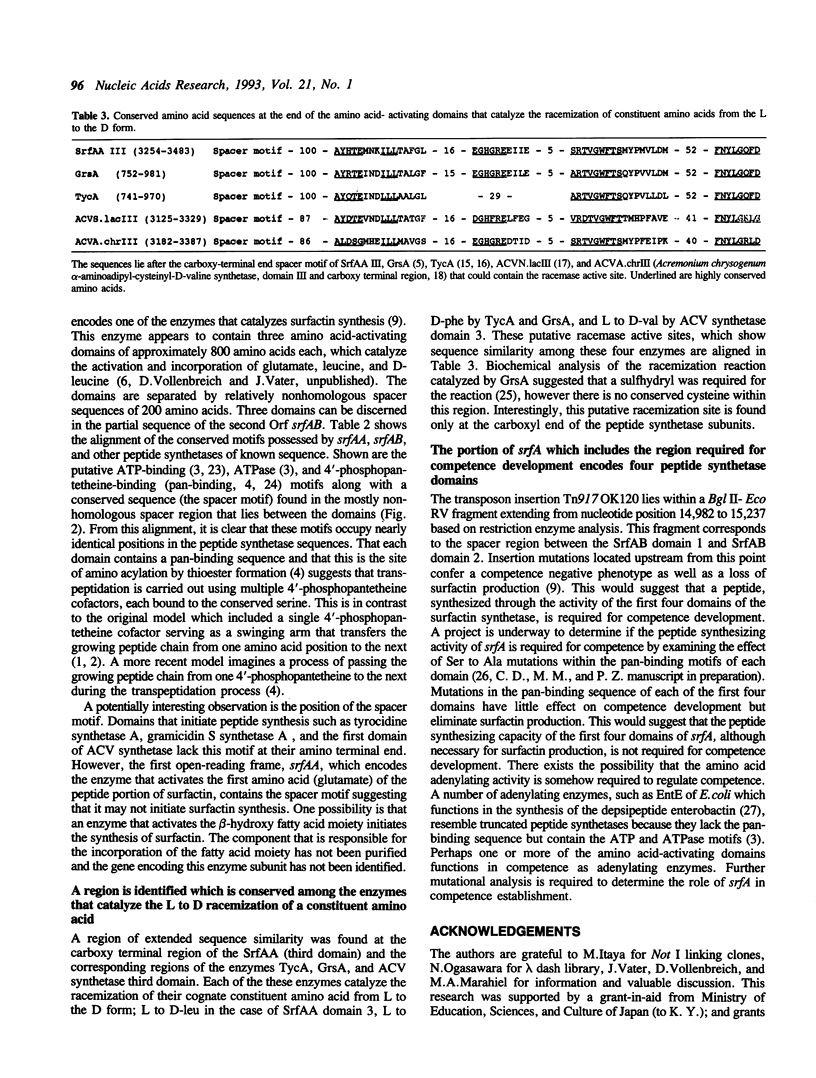
The nucleotide sequence of the 20,535 base pairs of the 5' end of the srfA operon, containing the region required for competence development, was determined. This included the srfA promoter region, the first open reading frame, srfAA, encoding surfactin synthetase I and part of the second open reading frame, srfAB, encoding surfactin synthetase II. Three amino acid-activating domains characteristic of those found in peptide synthetases could be discerned in both srfAA (activating Glu, Leu and D-Leu) and srfAB (activating Val, Asp, and D-Leu). The presence of a conserved spacer motif in the amino-terminal end of srfAA suggests that the srfAA product may not initiate surfactin synthesis. The portion of srfA that contains the region required for competence is composed of srfAA and the first amino acid-activating domain of srfAB.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Coque J. J., Martín J. F., Calzada J. G., Liras P. The cephamycin biosynthetic genes pcbAB, encoding a large multidomain peptide synthetase, and pcbC of Nocardia lactamdurans are clustered together in an organization different from the same genes in Acremonium chrysogenum and Penicillium chrysogenum. Mol Microbiol. 1991 May;5(5):1125–1133. doi: 10.1111/j.1365-2958.1991.tb01885.x. [DOI] [PubMed] [Google Scholar]
- Dubnau D. Genetic competence in Bacillus subtilis. Microbiol Rev. 1991 Sep;55(3):395–424. doi: 10.1128/mr.55.3.395-424.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell D. H., Mikesell P., Actis L. A., Crosa J. H. A regulatory gene, angR, of the iron uptake system of Vibrio anguillarum: similarity with phage P22 cro and regulation by iron. Gene. 1990 Jan 31;86(1):45–51. doi: 10.1016/0378-1119(90)90112-5. [DOI] [PubMed] [Google Scholar]
- Gutiérrez S., Díez B., Montenegro E., Martín J. F. Characterization of the Cephalosporium acremonium pcbAB gene encoding alpha-aminoadipyl-cysteinyl-valine synthetase, a large multidomain peptide synthetase: linkage to the pcbC gene as a cluster of early cephalosporin biosynthetic genes and evidence of multiple functional domains. J Bacteriol. 1991 Apr;173(7):2354–2365. doi: 10.1128/jb.173.7.2354-2365.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itaya M., Tanaka T. Complete physical map of the Bacillus subtilis 168 chromosome constructed by a gene-directed mutagenesis method. J Mol Biol. 1991 Aug 5;220(3):631–648. doi: 10.1016/0022-2836(91)90106-g. [DOI] [PubMed] [Google Scholar]
- Kanda M., Hori K., Kurotsu T., Miura S., Yamada Y., Saito Y. Sulfhydryl groups related to the catalytic activity of gramicidin S synthetase 1 of Bacillus brevis. J Biochem. 1981 Sep;90(3):765–771. doi: 10.1093/oxfordjournals.jbchem.a133531. [DOI] [PubMed] [Google Scholar]
- Kleinkauf H., von Döhren H. Nonribosomal biosynthesis of peptide antibiotics. Eur J Biochem. 1990 Aug 28;192(1):1–15. doi: 10.1111/j.1432-1033.1990.tb19188.x. [DOI] [PubMed] [Google Scholar]
- Krätzschmar J., Krause M., Marahiel M. A. Gramicidin S biosynthesis operon containing the structural genes grsA and grsB has an open reading frame encoding a protein homologous to fatty acid thioesterases. J Bacteriol. 1989 Oct;171(10):5422–5429. doi: 10.1128/jb.171.10.5422-5429.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köster W. L., Actis L. A., Waldbeser L. S., Tolmasky M. E., Crosa J. H. Molecular characterization of the iron transport system mediated by the pJM1 plasmid in Vibrio anguillarum 775. J Biol Chem. 1991 Dec 15;266(35):23829–23833. [PubMed] [Google Scholar]
- Lipmann F. Bacterial production of antibiotic polypeptides by thiol-linked synthesis on protein templates. Adv Microb Physiol. 1980;21:227–266. doi: 10.1016/s0065-2911(08)60357-4. [DOI] [PubMed] [Google Scholar]
- Mittenhuber G., Weckermann R., Marahiel M. A. Gene cluster containing the genes for tyrocidine synthetases 1 and 2 from Bacillus brevis: evidence for an operon. J Bacteriol. 1989 Sep;171(9):4881–4887. doi: 10.1128/jb.171.9.4881-4887.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano M. M., Magnuson R., Myers A., Curry J., Grossman A. D., Zuber P. srfA is an operon required for surfactin production, competence development, and efficient sporulation in Bacillus subtilis. J Bacteriol. 1991 Mar;173(5):1770–1778. doi: 10.1128/jb.173.5.1770-1778.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano M. M., Marahiel M. A., Zuber P. Identification of a genetic locus required for biosynthesis of the lipopeptide antibiotic surfactin in Bacillus subtilis. J Bacteriol. 1988 Dec;170(12):5662–5668. doi: 10.1128/jb.170.12.5662-5668.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano M. M., Xia L. A., Zuber P. Transcription initiation region of the srfA operon, which is controlled by the comP-comA signal transduction system in Bacillus subtilis. J Bacteriol. 1991 Sep;173(17):5487–5493. doi: 10.1128/jb.173.17.5487-5493.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusnak F., Sakaitani M., Drueckhammer D., Reichert J., Walsh C. T. Biosynthesis of the Escherichia coli siderophore enterobactin: sequence of the entF gene, expression and purification of EntF, and analysis of covalent phosphopantetheine. Biochemistry. 1991 Mar 19;30(11):2916–2927. doi: 10.1021/bi00225a027. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraste M., Sibbald P. R., Wittinghofer A. The P-loop--a common motif in ATP- and GTP-binding proteins. Trends Biochem Sci. 1990 Nov;15(11):430–434. doi: 10.1016/0968-0004(90)90281-f. [DOI] [PubMed] [Google Scholar]
- Schlumbohm W., Stein T., Ullrich C., Vater J., Krause M., Marahiel M. A., Kruft V., Wittmann-Liebold B. An active serine is involved in covalent substrate amino acid binding at each reaction center of gramicidin S synthetase. J Biol Chem. 1991 Dec 5;266(34):23135–23141. [PubMed] [Google Scholar]
- Smith D. J., Earl A. J., Turner G. The multifunctional peptide synthetase performing the first step of penicillin biosynthesis in Penicillium chrysogenum is a 421,073 dalton protein similar to Bacillus brevis peptide antibiotic synthetases. EMBO J. 1990 Sep;9(9):2743–2750. doi: 10.1002/j.1460-2075.1990.tb07461.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich C., Kluge B., Palacz Z., Vater J. Cell-free biosynthesis of surfactin, a cyclic lipopeptide produced by Bacillus subtilis. Biochemistry. 1991 Jul 2;30(26):6503–6508. doi: 10.1021/bi00240a022. [DOI] [PubMed] [Google Scholar]
- Weckermann R., Fürbass R., Marahiel M. A. Complete nucleotide sequence of the tycA gene coding the tyrocidine synthetase 1 from Bacillus brevis. Nucleic Acids Res. 1988 Dec 23;16(24):11841–11841. doi: 10.1093/nar/16.24.11841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuber P. Non-ribosomal peptide synthesis. Curr Opin Cell Biol. 1991 Dec;3(6):1046–1050. doi: 10.1016/0955-0674(91)90127-k. [DOI] [PubMed] [Google Scholar]
- van Sinderen D., Withoff S., Boels H., Venema G. Isolation and characterization of comL, a transcription unit involved in competence development of Bacillus subtilis. Mol Gen Genet. 1990 Dec;224(3):396–404. doi: 10.1007/BF00262434. [DOI] [PubMed] [Google Scholar]


