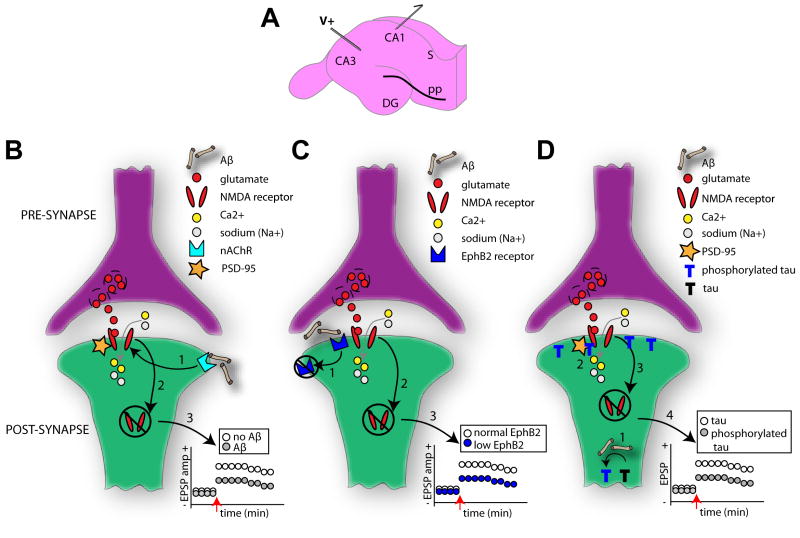
Figure 2. Multiple mechanistic factors contribute to synaptic dysfunction.
(A) Diagram of the hippocampal slice preparation which is commonly used to elucidate mechanisms of learning and memory. Regions CA1, CA3, dentate gyrus (DG) and subiculum (S) are indicated. In this example, a traditional paradigm with electric stimulation (V+) of the Schaffer collaterals and postsynaptic recordings (outgoing arrow) from the hippocampal CA1 pyramidal cells is depicted. Long-term potentiation (LTP) in these synapses is NMDAR (N-methyl-d-aspartic acid receptor) dependent and subject of vast research regarding AD-related cognitive deficits [182]. Several notable models regarding AD mechanisms on cellular learning and memory exist. NMDARs normally allow the influx of calcium (Ca2+) and sodium (Na+) ions upon the binding of glutamate. In one model (B), however, the binding of soluble Aβ to postsynaptic membrane-bound α-7 nicotinic (nAChR) receptors (1) results in the eventual destabilization of the neuronal PDZ protein, PSD-95, which anchors the NMDARs to the synaptic membrane. This destabilization of the NMDAR yields its ultimate internalization (2). This internalization of membrane bound NMDARs reduces the strength of the synapse (for instance less long-term potentiation LTP) (3). In related model (C) [87], Aβ oligomers bind to the receptor tyrosine kinase EphB2 (1), preventing the normal EphB2 – NMDA receptor interaction known important for stabilization of NMDA receptors on the membrane and induction of LTP [88]. EphB2-dependent loss of surface NMDARs (2) thereby reduces synaptic strength (3). Tau is also thought to impact synaptic strength in an Aβ-dependent manner (D) [55]. Hyperphosphorylation of tau, due to Aβ, results in the aggregation of phosphorylated tau in dendritic spines (1). This may lead to reduced stabilization of NMDARs to the PSD complex (2) and ultimately their internalization (3). In this manner phosphorylated tau may reduce synaptic strength (4). Across all of these models the enhanced NMDAR internalization will entail reduced surface expression of AMPA receptors (α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid) and a major change in synaptic efficacy, and ultimately spine loss [89].