Figure 4. Curcumin inhibits radiation-induced activation of proteins upstream of NF-κB.
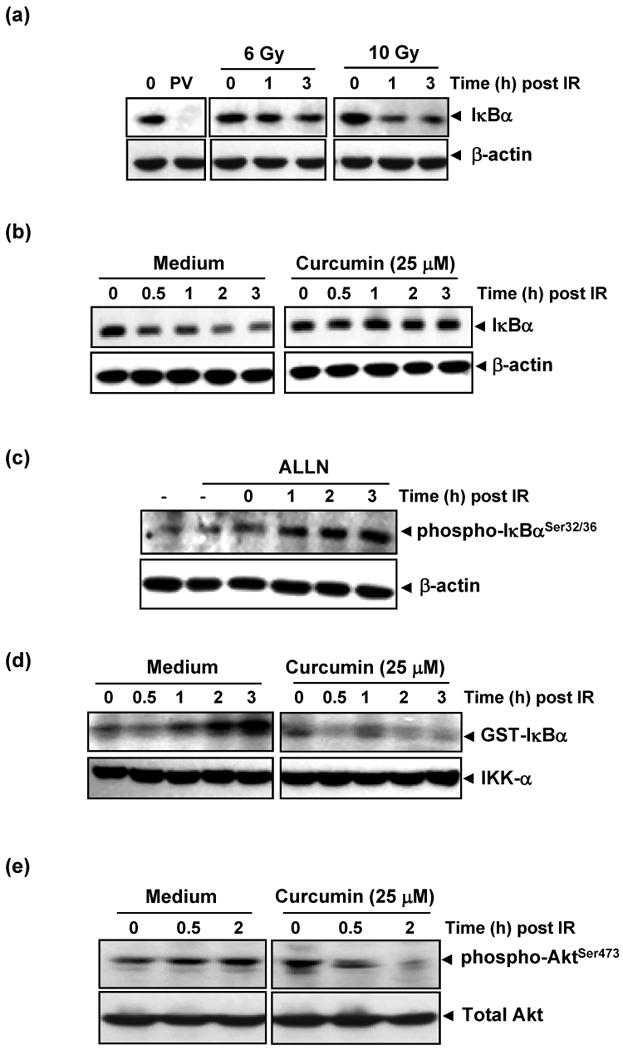
(a) Radiation induced IκBα degradation. HCT116 cells were exposed to 6 or 10 Gy of radiation and incubated for the indicated times. Pervanadate (PV; 50μM), a protein tyrosine phosphatase inhibitor, which induces IκBα degradation via tyrosine phosphorylation was positive control. Cytoplasmic extracts were prepared and subjected to immunoblotting using anti-IκBα antibody. (b) Pre-treatment with curcumin abrogated radiation-induced degradation of IκBα. HCT116 cells were incubated with 25 μM curcumin for 4 h prior to radiation (10 Gy) and cytoplasmic extracts were immunoblotted as described above. (c) Radiation induced phosphorylation of IκBα. HCT116 cells were incubated with 50 μg/ml ALLN for 30 min, exposed to radiation (10 Gy), and cytoplasmic extracts obtained at different time points were subjected to immunoblot analysis using phospho-specific anti-IκBα antibody (Ser32/36) (d) Curcumin inhibited radiation-induced IKK activation. HCT116 cells were pre-treated with or without 25 μM curcumin for 4 h and the IKK complex from whole-cell extracts was precipitated with antibody against IKK-α. IKK activity was analyzed by an immune complex kinase assay. The fusion protein GST-IκBα (glutathione S-transferase-IκBα) that was subsequently immunoblotted serves as a substrate for IKK. (e) Pre-treatment with curcumin inhibited radiation-induced activation of Akt. In experiments similar to those above, cytoplasmic extracts of HCT116 cells treated with and without curcumin prior to radiation were immunoblotted with anti phospho-Akt Ser473 antibody.
