Abstract
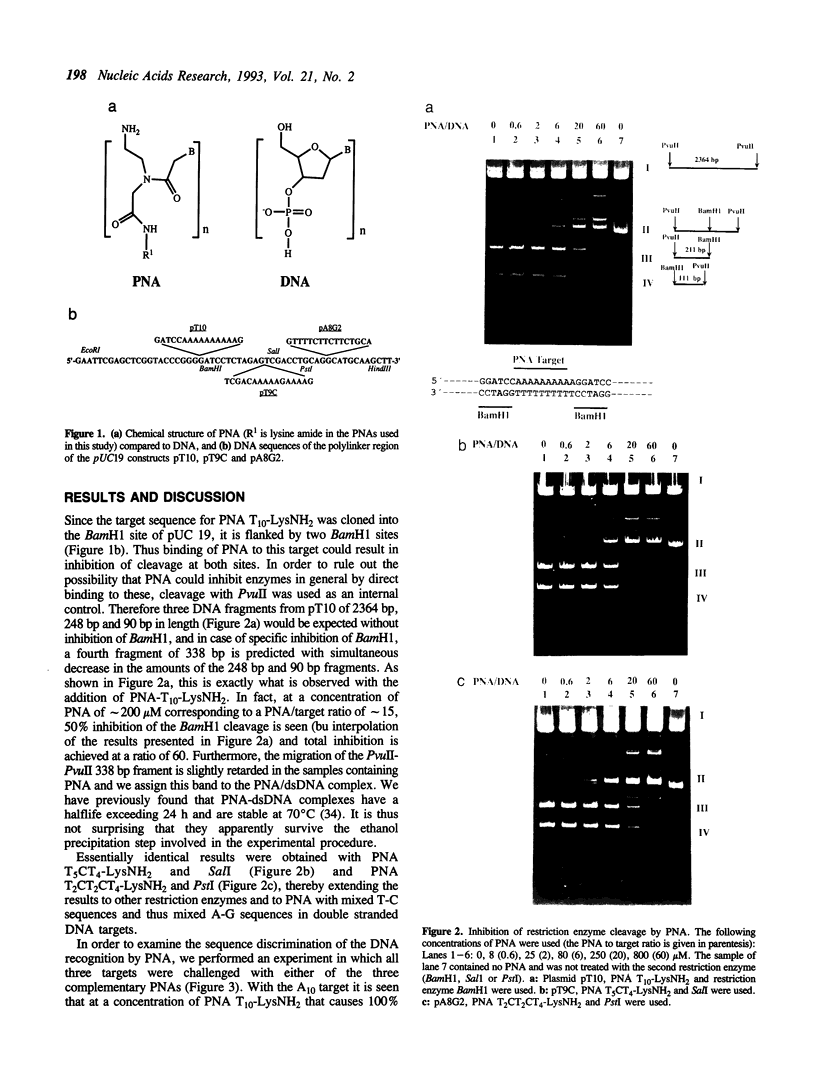
Plasmids containing double-stranded 10-mer PNA (peptide nucleic acid chimera) targets proximally flanked by two restriction enzyme sites were challenged with the complementary PNA or PNAs having one or two mismatches, and the effect on the restriction enzyme cleavage of the flanking sites was assayed. The following PNAs were used: T10-LysNH2, T5CT4-LysNH2 and T2CT2CT4-LysNH2 and the corresponding targets cloned into pUC 19 were flanked by BamH1, Sal1 or Pstl sites, respectively. In all cases it was found that complete inhibition of restriction enzyme cleavage was obtained with the complementary PNA, a significantly reduced effect was seen with a PNA having one mismatch, and no effect was seen with a PNA having two mismatches. These results show that PNA can be used as sequence specific blockers of DNA recognizing proteins.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birg F., Praseuth D., Zerial A., Thuong N. T., Asseline U., Le Doan T., Hélène C. Inhibition of simian virus 40 DNA replication in CV-1 cells by an oligodeoxynucleotide covalently linked to an intercalating agent. Nucleic Acids Res. 1990 May 25;18(10):2901–2908. doi: 10.1093/nar/18.10.2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dervan P. B. Design of sequence-specific DNA-binding molecules. Science. 1986 Apr 25;232(4749):464–471. doi: 10.1126/science.2421408. [DOI] [PubMed] [Google Scholar]
- Duval-Valentin G., Thuong N. T., Hélène C. Specific inhibition of transcription by triple helix-forming oligonucleotides. Proc Natl Acad Sci U S A. 1992 Jan 15;89(2):504–508. doi: 10.1073/pnas.89.2.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorova O. S., Knorre D. G., Podust L. M., Zarytova V. F. Complementary addressed modification of double-stranded DNA within a ternary complex. FEBS Lett. 1988 Feb 15;228(2):273–276. doi: 10.1016/0014-5793(88)80014-0. [DOI] [PubMed] [Google Scholar]
- François J. C., Saison-Behmoaras T., Barbier C., Chassignol M., Thuong N. T., Hélène C. Sequence-specific recognition and cleavage of duplex DNA via triple-helix formation by oligonucleotides covalently linked to a phenanthroline-copper chelate. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9702–9706. doi: 10.1073/pnas.86.24.9702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- François J. C., Saison-Behmoaras T., Chassignol M., Thuong N. T., Helene C. Sequence-targeted cleavage of single- and double-stranded DNA by oligothymidylates covalently linked to 1,10-phenanthroline. J Biol Chem. 1989 Apr 5;264(10):5891–5898. [PubMed] [Google Scholar]
- François J. C., Saison-Behmoaras T., Thuong N. T., Hélène C. Inhibition of restriction endonuclease cleavage via triple helix formation by homopyrimidine oligonucleotides. Biochemistry. 1989 Dec 12;28(25):9617–9619. doi: 10.1021/bi00451a011. [DOI] [PubMed] [Google Scholar]
- Goodchild J. Conjugates of oligonucleotides and modified oligonucleotides: a review of their synthesis and properties. Bioconjug Chem. 1990 May-Jun;1(3):165–187. doi: 10.1021/bc00003a001. [DOI] [PubMed] [Google Scholar]
- Hanvey J. C., Shimizu M., Wells R. D. Site-specific inhibition of EcoRI restriction/modification enzymes by a DNA triple helix. Nucleic Acids Res. 1990 Jan 11;18(1):157–161. doi: 10.1093/nar/18.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausheer F. H., Singh U. C., Saxe J. D., Colvin O. M., T'su P. O. Can oligonucleoside methylphosphonates form a stable triplet with a double DNA helix? Anticancer Drug Des. 1990 May;5(2):159–167. [PubMed] [Google Scholar]
- Hélène C. The 1988 Walter Hubert lecture. Artificial control of gene expression by oligodeoxynucleotides covalently linked to intercalating agents. Br J Cancer. 1989 Aug;60(2):157–160. doi: 10.1038/bjc.1989.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hélène C., Thuong N. T. Control of gene expression by oligonucleotides covalently linked to intercalating agents. Genome. 1989;31(1):413–421. doi: 10.1139/g89-062. [DOI] [PubMed] [Google Scholar]
- Hélène C., Toulmé J. J. Specific regulation of gene expression by antisense, sense and antigene nucleic acids. Biochim Biophys Acta. 1990 Jun 21;1049(2):99–125. doi: 10.1016/0167-4781(90)90031-v. [DOI] [PubMed] [Google Scholar]
- Le Doan T., Perrouault L., Praseuth D., Habhoub N., Decout J. L., Thuong N. T., Lhomme J., Hélène C. Sequence-specific recognition, photocrosslinking and cleavage of the DNA double helix by an oligo-[alpha]-thymidylate covalently linked to an azidoproflavine derivative. Nucleic Acids Res. 1987 Oct 12;15(19):7749–7760. doi: 10.1093/nar/15.19.7749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyamichev V. I., Mirkin S. M., Frank-Kamenetskii M. D., Cantor C. R. A stable complex between homopyrimidine oligomers and the homologous regions of duplex DNAs. Nucleic Acids Res. 1988 Mar 25;16(5):2165–2178. doi: 10.1093/nar/16.5.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mergny J. L., Duval-Valentin G., Nguyen C. H., Perrouault L., Faucon B., Rougée M., Montenay-Garestier T., Bisagni E., Hélène C. Triple helix-specific ligands. Science. 1992 Jun 19;256(5064):1681–1684. doi: 10.1126/science.256.5064.1681. [DOI] [PubMed] [Google Scholar]
- Moser H. E., Dervan P. B. Sequence-specific cleavage of double helical DNA by triple helix formation. Science. 1987 Oct 30;238(4827):645–650. doi: 10.1126/science.3118463. [DOI] [PubMed] [Google Scholar]
- Nielsen P. E., Egholm M., Berg R. H., Buchardt O. Sequence-selective recognition of DNA by strand displacement with a thymine-substituted polyamide. Science. 1991 Dec 6;254(5037):1497–1500. doi: 10.1126/science.1962210. [DOI] [PubMed] [Google Scholar]
- Perrouault L., Asseline U., Rivalle C., Thuong N. T., Bisagni E., Giovannangeli C., Le Doan T., Hélène C. Sequence-specific artificial photo-induced endonucleases based on triple helix-forming oligonucleotides. Nature. 1990 Mar 22;344(6264):358–360. doi: 10.1038/344358a0. [DOI] [PubMed] [Google Scholar]
- Stein C. A., Cohen J. S. Oligodeoxynucleotides as inhibitors of gene expression: a review. Cancer Res. 1988 May 15;48(10):2659–2668. [PubMed] [Google Scholar]
- Strobel S. A., Dervan P. B. Single-site enzymatic cleavage of yeast genomic DNA mediated by triple helix formation. Nature. 1991 Mar 14;350(6314):172–174. doi: 10.1038/350172a0. [DOI] [PubMed] [Google Scholar]
- Strobel S. A., Dervan P. B. Site-specific cleavage of a yeast chromosome by oligonucleotide-directed triple-helix formation. Science. 1990 Jul 6;249(4964):73–75. doi: 10.1126/science.2195655. [DOI] [PubMed] [Google Scholar]
- Takasugi M., Guendouz A., Chassignol M., Decout J. L., Lhomme J., Thuong N. T., Hélène C. Sequence-specific photo-induced cross-linking of the two strands of double-helical DNA by a psoralen covalently linked to a triple helix-forming oligonucleotide. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5602–5606. doi: 10.1073/pnas.88.13.5602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trung Le Doan, Perrouault L., Chassignol M., Nguyen T. T., Hélène C. Sequence-targeted chemical modifications of nucleic acids by complementary oligonucleotides covalently linked to porphyrins. Nucleic Acids Res. 1987 Nov 11;15(21):8643–8659. doi: 10.1093/nar/15.21.8643. [DOI] [PMC free article] [PubMed] [Google Scholar]