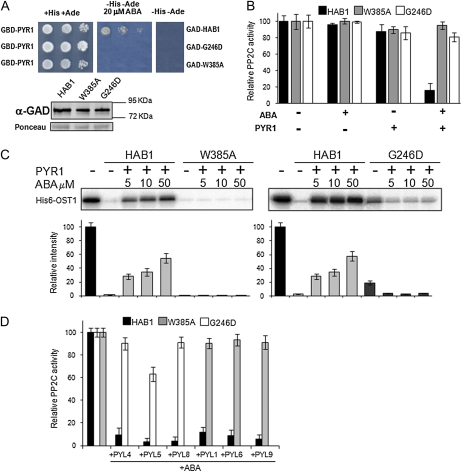
Figure 4.
The hab1W385A and hab1G246D PP2Cs are refractory to inhibition by PYR1 and dephosphorylate OST1 in the presence of ABA and PYR1. A, The HAB1 mutations Trp-385Ala and Gly-246Asp abolish the interaction of the PP2C and PYR1 in a Y2H assay. Immunoblot analysis using antibody against the Gal4 activation domain (GAD) is shown to verify the expression of the different fusion proteins. Ponceau staining from a representative yeast protein is shown as loading control. GBD, Gal4 binding domain. B, Phosphatase activity of HAB1, hab1W385A, and hab1G246D proteins was measured in vitro using pNPP as substrate in the absence or presence of PYR1 and ABA, as indicated. Assays were performed in a 100 μL reaction volume containing 2 μm phosphatase and, when indicated, 4 μm HIS6-PYR1 and 1 μm (+)-ABA. Data are averages ± sd from three independent experiments. C, In vitro OST1 kinase activity in the presence of wild-type and mutated versions of HAB1, PYR1, and ABA, as indicated. The autoradiography shows the level of autophosphorylation of OST1 in each reaction. The graphs show the quantitative analysis of the autoradiogram. D, hab1W385A and hab1G246D proteins are resistant to ABA-mediated inhibition by different PYR/PYLs. The assay was performed as described in B. [See online article for color version of this figure.]