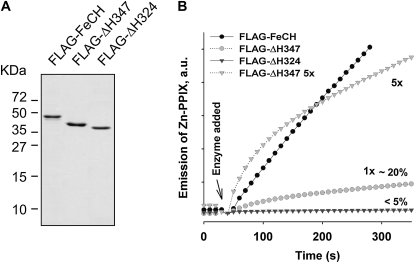
Figure 4.
Full-length and truncated FeCH enzymes purified from Synechocystis. A, FLAG-tagged full-length and truncated FeCHs were purified under native conditions on the anti-FLAG affinity gel, separated by SDS electrophoresis, and stained by Coomassie Brilliant Blue; approximately 0.5 μg of protein was loaded in each line. B, In vitro activities of purified FeCH enzymes as determined by continuous spectrofluorometric assay. Activities were monitored as an increase in fluorescence of zinc-PPIX using excitation and emission wavelengths of 420 and 590 nm, respectively; 1x indicates that approximately 0.1 μg of enzyme was assayed. Relative activities of truncated enzymes in comparison with the full-length FeCH (100%) are also indicated.