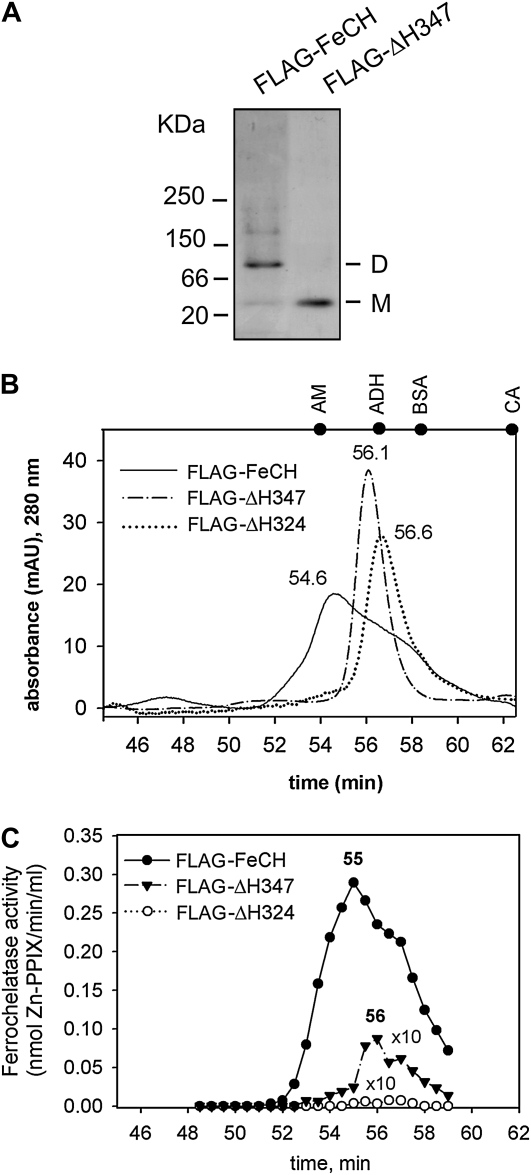
Figure 5.
Aggregation state of purified FeCHs. A, Approximately 0.5 μg of the purified wild-type and truncated FeCH was separated by nondenaturing electrophoresis and stained by Coomassie Brilliant Blue. M and D indicate the positions of FeCH monomer and dimer, respectively. B, Gel filtration of the FLAG-FeCH and truncated FeCH enzymes on the BioSep SEC-S3000 column. Approximately 1 μg of each protein was separated. Positions of standards are shown at the top of the graph: AM = β-amylase (200 kD); ADH = alcohol dehydrogenase (150 kD); BSA = bovine serum albumin (66 kD); CA = carbonic anhydrase (29 kD). C, FeCH activity in 0.5-min fractions as eluted from the gel filtration column during separation of purified FeCH enzymes (described in B). The whole volume of each fraction (75 μL) was assayed immediately after elution in an in vitro FeCH assay. Obtained values for truncated enzymes were multiplied 10-fold to compare activity profiles.