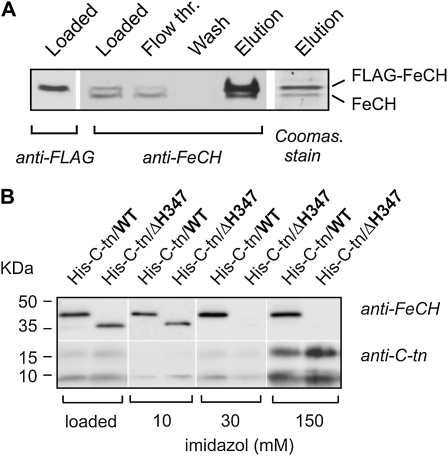
Figure 6.
A, Purification of the FLAG-FeCH in a complex with nontagged FeCH. FLAG-FeCH was purified from the FLAG-hemH strain possessing both tagged and nontagged forms of this enzyme; purification was carried out under native conditions on the anti-FLAG affinity gel as described in “Materials and Methods.” Each purification step was separated by SDS electrophoresis and blotted, and both FeCH forms were detected by anti-FeCH antibody; eluted proteins separated by SDS electrophoresis were also stained by Coomassie Brilliant Blue. The position of the FLAG-FeCH was resolved by anti-FLAG antibody. B, Purification of the FeCH C-terminal fragment and its copurification with the full-length FeCH and the ΔH347-FeCH lacking the CAB domain. The 63-residue-long C-terminal fragment of Synechocystis FeCH was expressed as a His-tagged protein (His-C-tn) in the wild type (WT) and in the ΔH347 backgrounds and purified from both strains using Ni2+ affinity chromatography. As the polyclonal anti-FeCH antibody recognized the C-terminal parts of the protein only weakly, the His-C-tn was detected using an antibody raised against a synthetic peptide corresponding to region II of the Synechocystis FeCH (amino acids 333–348). The amount of membrane protein loaded for each sample corresponded to 150 μL of cells at OD750 = 1, 1/50th the volume of each washing step (20 μL from 1 mL), and 1/25th the total elution volume. The imidazole elution is described in “Materials and Methods.”