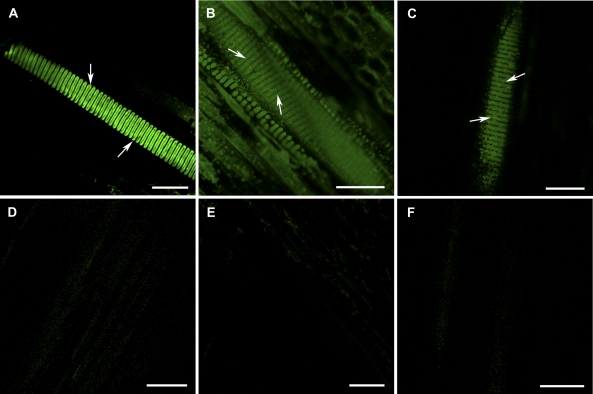
Figure 1.
Effectiveness of the immunohistochemical technique with cell wall antibodies and CLSM in detecting some polysaccharide compositions in intervessel PMs from the tangential sectional views of secondary xylem. A to C, Secondary xylem tissue treated with primary antibody and the corresponding secondary antibody. A, Strong fluorescent signal was detected in the intervessel PMs (arrows) of grapevine var. Chardonnay when incubated with CCRC-M1 in a 3-fold dilution of its hybridoma supernatant and FITC-conjugated goat antimouse IgG in a 200-fold dilution. B, Obvious fluorescent signal from the intervessel PMs (arrows) in grapevine var. Riesling incubated with JIM5 and FITC-conjugated rabbit antirat IgG, which were in 10- and 100-fold dilutions, respectively. C, Fluorescence from the intervessel PMs (arrows) in grapevine var. Riesling incubated with JIM7 and FITC-conjugated rabbit antirat IgG, which were in 10- and 100-fold dilutions, respectively. D to F, Secondary xylem tissue of grapevine var. Chardonnay vines in experimental controls, which did not experience the incubation with a primary antibody, a matched secondary antibody, or both. Fluorescent signal was picked up at the same level of amplification as in A to C. D, Xylem tissue not incubated with CCRC-M1 but with FITC-conjugated goat antimouse IgG. No obvious fluorescent signals were detected. E, Xylem tissue incubated with CCRC-M1 but without FITC-conjugated goat antimouse IgG showed no detectable fluorescence. F, No obvious fluorescence from the xylem tissue not incubated with either CCRC-M1 or FITC-conjugated goat antimouse IgG. Bar in each section equals 50 μm.