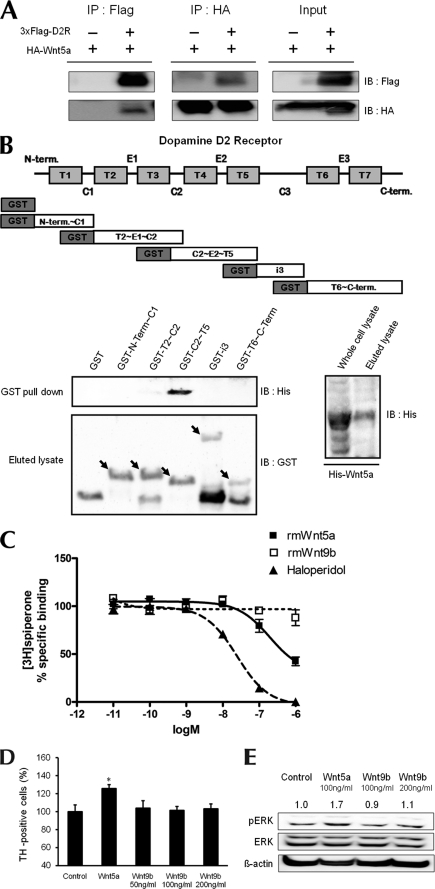
FIGURE 3.
Interaction of Wnt5a with D2R. A, immunoprecipitation showing an association between D2R and Wnt5a. Protein samples were obtained from transfecting p3×FLAG-myc-CMVTM-26-D2R and pLNCX-HA-Wnt5a into HEK293T cells. B, schematic diagram of five domain fragments of D2R (amino acids 1–71, 72–151, 131–210, 211–374, 375–444). Domain fragments are represented as GST-N-Term∼C1, GST-T2∼C2, GST-C2∼T5, GST-i3, GST-T6∼C-Term, respectively. GST pulldown assays used bacterially expressed GST-D2R mutants and His-Wnt5a. Pulldown proteins were analyzed by immunoblotting with His antibody. The left bottom panel shows GST and GST-D2R mutants eluted with glutathione-Sepharose 4B and immunoblotted with GST antibody. The right bottom panel shows His-Wnt5a purified on nickel-nitrilotriacetic acid-agarose and immunoblotted with His antibody. C, displacement of spiperone biding by haloperidol or by Wnt5a. The binding capacities of rmWnt5a, rmWnt9b, and haloperidol were evaluated by inhibition of [3H]spiperone binding to the D2R following treatment with rmWnt5a and haloperidol. Total binding was assessed in the absence of compounds; nonspecific binding was measured in the presence of 5 μm butaclamol. Values are mean ± S.E. of four independent experiments, each conducted in duplicate. In each of the experiments, the curves were fitted via nonlinear regression analysis using the one-site competition model (GraphPad Prism program). D, effect of treatment with Wnt5a (100 ng/ml), Wnt9b (50, 100, 200 ng/ml) on the numbers of TH neurons analyzed in mesencephalic neuronal cultures from WT mice. The mean values ± S.E. are shown for WT, n = 3. *, p < 0.05, ANOVA followed by a Newman-Keuls test for control versus drug-treated cells of WT mice. E, cells treated with 100 ng/ml Wnt5a and 100 ng/ml or 200 ng/ml Wnt9b. *, p < 0.05; **, p < 0.01; ***, p < 0.001, ANOVA followed by a Newman-Keuls test for control versus drug-treated cells. †, p < 0.05; ††, p < 0.01; †††, p < 0.001, unpaired Student's t test for WT and D2R−/− mice. Halo, haloperidol.