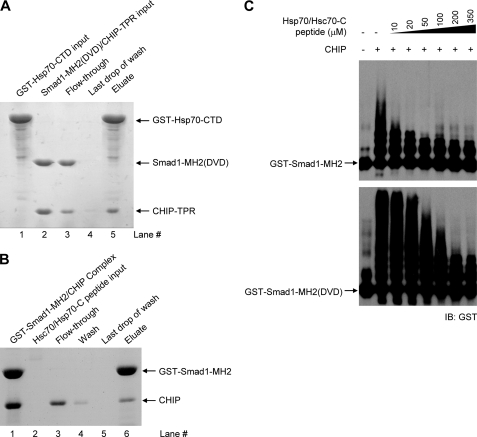
FIGURE 5.
Heat shock proteins and Smad1 compete for CHIP binding. A, disruption of the CHIP-Smad1 (DVD) complex by Hsp70-CTD is shown. Some 0.4 mg of recombinant GST-Hsp70-CTD was bound to 0.2 ml glutathione-Sepharose 4B resin (lane 1). The resin was washed five times with 1.0 ml of buffer to remove excess unbound Hsp70 or other contaminants. Then, 0.6 mg of non-tagged CHIP-Smad1 complex was allowed to flow through the resin (lanes 2 and 3). After extensive washing (lane 4), the bound proteins were eluted with 5 mm reduced glutathione (lane 5). All fractions were visualized by SDS-PAGE with Coomassie Blue staining. B, Hsp70/Hsc70-C peptide competes with Smad1 for CHIP binding. The complex of GST-Smad1 and CHIP was loaded to the resin followed by the addition of the chaperone peptide. The column was then washed extensively and eluted with reduced glutathione. Samples were visualized by SDS-PAGE with Coomassie Blue staining. C, heat shock protein antagonizing CHIP-mediated Smad1 ubiquitination is shown. In vitro ubiquitination assays were performed in the absence or presence of increasing amounts of Hsp70/Hsc70-C peptide. IB, immunoblot.