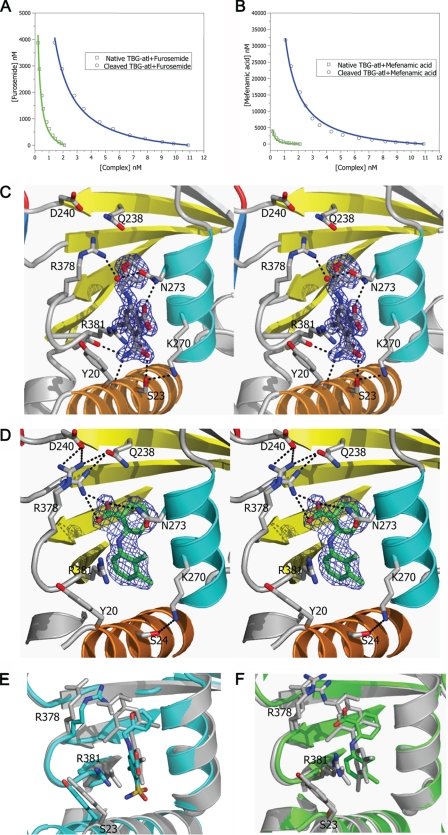
FIGURE 4.
Binding of T4 analogues to TBG. The binding affinities of furosemide (A) or mefenamic acid (B) toward TBG conformers were measured by competitive assay with titration of the analogues into a TBG/T4–6-CF mixed solution with the fluorescence signal monitored and fitted using the competitive binding equations II and III. The fitted curves are shown in blue (cTBG) or green (native TBG). C and D, stereo-view of the binding site of cTBG-furosemide complex (C) and cTBG-mefenamic acid (D). Electron density, contoured at 2.5 times the root mean square of the map for furosemide and 1 root mean square for mefenamic acid, is shown in blue mesh. Key residues are shown in sticks with carbon atoms are shown in green, nitrogen atoms are shown in blue, and oxygen atoms are shown in red. Furosemide (C) forms four hydrogen bonds with surrounding residues, but it has no interaction with Arg378. The side chain of Arg378 is largely solvent exposed with little direct interaction with the binding site. Whereas in the cTBG-mefenamic acid structure (D), Arg378 exiting in alternative conformations, forms ionic interactions with both the ligand and Asp240, similar to that of thyroxine (Fig. 2C). E and F, when these two structures are superimposed with that of cTBG-thyroxine (gray), it is apparent that the furosemide-binding site (E, light blue) is slightly smaller and the side chain of Arg378 shifts away from the s2/3B loop and will thus be insensitive to its shifts. The side chain of Arg378 of the cTBG-mefenamic acid structure (F, green), however, retains a similar position and hence responsiveness to the S-to-R change as that of cTBG-thyroxine.