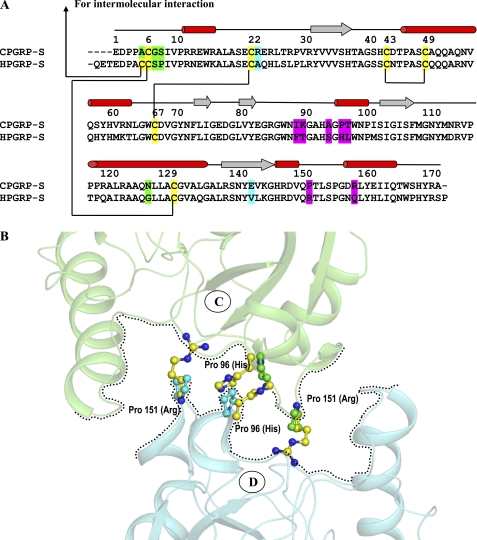
FIGURE 5.
A, sequence alignment of CPGRP-S and HPGRP-S is shown with elements of secondary structures on top. Cysteine residues are highlighted in yellow. The important residues at the A-B (green) and C-D (red) interfaces are compared with the corresponding residues in HPGRP-S. The differences indicate incompatibilities in HPGRP-S for making an oligomer as observed in CPGRP-S. B, the formation of a PAMPs-binding cleft at the interface of molecules C and D is hampered in HPGRP-S because four proline residues (Pro-C96, Pro-C151, Pro-D96, and Pro-D151) at the C-D interface in CPGRP-S are replaced with His-96 and Arg-151 in HPGRP-S.