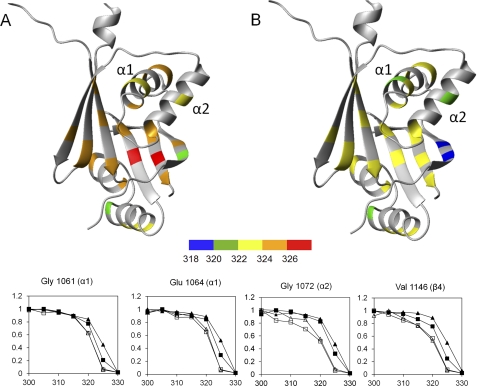
FIGURE 7.
Stability of the structural elements in the wild type and H1069Q-WND. A and B, midpoints of thermal denaturation curves were recorded by measuring volumes of the HSQC peaks of individual backbone amide groups in the folded forms of the wild type (A) and the H1069Q variant (B). Residues with large folding-dependent secondary chemical shifts that produced well separated peaks in the HSQC spectra (4) were chosen as reporter groups. C, relative peak volumes of several selected backbone amide groups in the wild-type WND (triangles) and the H1069Q-WND (squares) in the apo form (open symbols) and in the presence of 1 mm ATP (filled symbols) were recorded as a function of sample temperature (K).