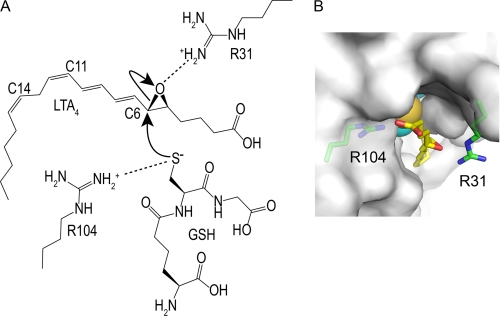
FIGURE 1.
Schematic representation of the proposed catalytic mechanism. A, the catalytic mechanism for LTC4 biosynthesis catalyzed by Arg-31 and Arg-104 was proposed from the crystal structure of LTC4S and the putative LTA4 binding model (35). In panel B the surface of the LTC4S trimer is in white. The side chain of Arg-31 was flexible in the crystal structure of this work, so the side chain of Arg-31 with the most common conformation in the crystal structure is presented as a plausible model (55). The buried GSH molecule is shown by the CPK (Corey Pauling Koltun) model. The epoxide group comes to the space between the Arg-31 and Arg-104. The space is the only place where the epoxide group can interact with the thiol group, because the thiol group of GSH is buried inside of the trimer. The epoxide group can bind in a productive manner there in which the epoxy carbon comes to the proximity of the thiol group and the epoxy oxygen resides at the opposite side of the thiol group, although the binding mode of LTA4 remains to be confirmed experimentally. The positively charged Arg-31 increases the electrophilicity of the C6 of LTA4 by forming a hydrogen bond with the epoxide oxygen, and the positively charged Arg-31 stabilizes the negatively charged alkoxide group, which forms from the epoxide group concomitantly with the propagation of the catalysis. Through the direct interaction between the guanidino side chain of Arg-104 and the thiol group of GSH, the Arg-104 decreases the pKa of the thiol group to the level where the thiol group becomes the activated species as a thiolate anion at physiological pH. Then, the resultant thiolate anion attacks the electrophilic C6 of LTA4.