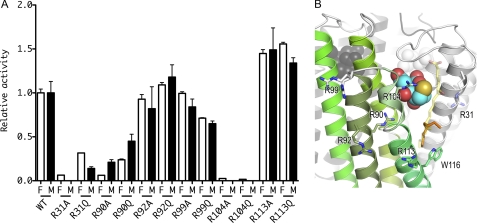
FIGURE 2.
Relative activities of the arginine mutants in comparison to WT LTC4S. A, relative activities of the arginine mutants in comparison to WT LTC4S are shown with S.D. The enzyme activities of the mutant enzymes with LTA4 and LTA4-Me were normalized to the enzyme activities of the WT LTC4S with LTA4 and LTA4-Me, respectively. The open and the closed bars depict the relative enzyme activities measured using LTA4 (F) and LTA4-Me (M), respectively. B, the arginine residues mutated in this work are shown by stick models. The ribbon model with green is a monomer in the LTC4S trimer, and the other two are shown in gray. The translucent stick model with yellow carbons represents the putative LTA4 binding model, and the alkyl chain of the dodecyl maltoside used for the modeling of the LTA4 is shown by the stick model with orange carbons. The conformation of the side chain of Arg-31 is the one most commonly observed (55). The side chain did not have a uniform conformation in the crystal structure. Therefore, the side chain of Arg-31 modeled was represented by the translucent stick model.