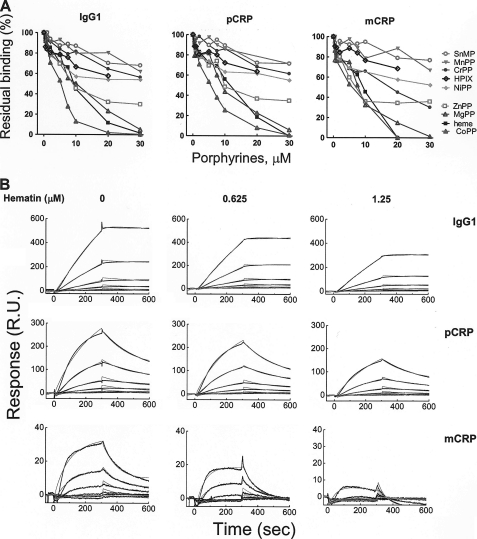
FIGURE 2.
Inhibition of the interaction of C1q with IgG1 and CRP by heme and different porphyrins. A, the inhibitory activity of different porphyrins on the binding of C1q to pCRP, mCRP, and IgG1 was assessed by real-time interaction analyses. C1q (0.1 μm) was exposed to increasing concentrations of metalloporphyrins (0–30 μm) and injected for 5 min over immobilized IgG, pCRP, and mCRP. The graph depicts the percentage of residual binding of C1q as a function of the porphyrin concentration. The binding of native C1q is considered as 100%. All stock solutions of metalloporphyrins were in dimethyl sulfoxide except Mg(II)PP, which was dissolved in water. B, real-time profiles of the interaction of native and hematin-exposed C1q to IgG1, pCRP, and mCRP. C1q at 0.1 μm was exposed to the indicated concentrations of hematin or to vehicle only. The profiles were generated by injecting increasing concentrations of C1q (5, 2.5, 1.25, 0.625, 0.312, and 0.156 nm) over sensor chips immobilized with IgG1, mCRP, and pCRP. The time-dependant binding of C1q in the indicated conditions is presented in resonance units (R.U.). The experimental binding curves (black lines) and curves generated by fitting the data to Langmuir binding with drifting baseline model (gray lines) are presented. SnMP, Sn(IV)MP; MgPP, Mg(II)PP; MnPP, Mn(III)PP; HPIX, hematoporphyrin IX; CrPP, Cr(IV)PP; NiPP, Ni(II)PP; CoPP, Co(III)PP.