Abstract
Introduction
The diagnosis and assessment of apical periodontitis by traditional periapical radiographs can be challenging and might yield false-negative results. The aim of this study was to determine whether interleukin-1beta (IL-1β) and dentin sialoprotein (DSP) in gingival crevicular fluid (GCF) can be used as biological markers for apical periodontitis.
Methods
Forty healthy patients with teeth diagnosed with apical periodontitis of pulpal origin were included in the study. GCF samples were obtained from the diseased tooth and from a healthy contralateral control tooth. Total protein concentration in each sample was determined by using the Bio-Rad protein assay. Enzyme-linked immunosorbent assay was used to analyze the concentration of IL-1β and DSP in the samples.
Results
Protein content of the GCF was statistically significantly higher in the disease group compared with the control group. The levels of IL-1β and DSP were not statistically different between disease and control groups.
Conclusions
Although this study was unable to demonstrate a significantly higher level of IL-1β or DSP in the GCF of teeth with apical periodontitis, the observed presence of a significantly higher level of total protein in the GCF of diseased teeth suggests the possible role of total protein level as a marker for periapical disease.
Keywords: Apical periodontitis, biological marker, gingival crevicular fluid
The inflammatory periapical lesion is a common sequela of infected pulp necrosis and manifests itself as the host defense response to microbial challenge emanating from the pulp canal system. Numerous cell types, including polymorphonuclear leukocytes, T and B lymphocytes, macrophages, and plasma cells, have been identified in periapical lesions (1). These inflammatory cells, especially macrophages, mediate the immunologic response seen in apical periodontitis (2). Bone resorption seen in periradicular lesions is mainly caused by the production of interleukin-1beta (IL-1β) by macrophages (3, 4) and tumor necrosis factor–β by T lymphocytes (5). Il-1β is commonly found in human periapical lesions (6).
Dentin resorption can also occur during the development of apical periodontitis (7). Dentin sialoprotein (DSP) is a dentin non-collagenous protein involved in the mineralization of predentin into dentin. DSP was found in the gingival crevicular fluid (GCF) of patients presenting with external apical root resorption caused by orthodontic movement (8). DSP was once thought to be a dentin-specific protein, but its expression was also demonstrated in bone tissue, although in much lower levels than in dentin (9). Therefore, it is possible that DSP detected in GCF is not exclusively from dentin resorption.
Currently, the presence or absence of apical periodontitis is determined by clinical and radiographic examination. Because there is a relatively low incidence of clinical signs and symptoms associated with periradicular periodontitis (10), the diagnosis is established primarily by radiographic findings in association with pulp vitality tests. Clinical symptoms are present in only approximately 18%–24% of teeth with radiographic evidence of apical periodontitis (10, 11). The limitations of radiographic examination in detecting the presence of apical periodontitis are well-known and are related to the amount of bone loss caused by the lesion, the spread of bone resorption into the cortical bone, location in the jaw, and operator variability in radiographic interpretation (12, 13). Recent studies demonstrated that cone beam computed tomography (CBCT) is more accurate in detecting apical periodontitis compared with conventional radiographs (14–19). Because both the cost of CBCT and radiation exposure continue to decrease, its use for the assessment of periapical healing will likely become more common.
Peripheral body fluids such as GCF are often used as identity markers of acute and chronic inflammation because the composition of these fluids might change as a result of their proximity to an inflammatory focus. GCF is the inflammatory exudate that can be collected at the gingival crevice. Collection of GCF is simple and presents minimal risk to the patient. Biological markers such as inflammatory mediators and neuropeptides were detected in the GCF of patients with periodontal disease (20) and root resorption caused by orthodontic treatment (8). Increased levels of substance P, neurokinin A, and IL-8 were found in the GCF of patients with acute irreversible pulpitis (21, 22). In the presence of active disease associated with apical periodontitis, inflammatory mediators and dentin proteins that are released in the periapical tissues might diffuse through the periodontal ligament and subsequently into the gingival crevice.
The objective of this study was to test the hypothesis that higher levels of IL-1β and DSP would be detected in the GCF of teeth diagnosed with apical periodontitis compared with healthy control teeth in the same patient. The long-term goal is to develop a reliable, inexpensive, and noninvasive test that could be used as an adjunct to currently used diagnostic tools to detect the presence of active periapical inflammation.
Materials and Methods
Forty patients were recruited from the pool of patients seeking routine or emergency treatment in the Postgraduate Endodontics Clinic of the University of Illinois at Chicago (UIC). The UIC Institutional Review Board approved the study. Written and verbal informed consent was obtained from each patient.
The inclusion/exclusion criteria for this study were as follows. Participants must be 18 years old or older with an unremarkable medical history and radiographically evident apical periodontitis on a restorable single or multirooted tooth. Patients undergoing first time root canal therapy (RCT), retreatment RCT, and surgical RCT were included. Pulp vitality testing was performed on teeth that had not been previously treated to confirm pulpal necrosis. Patients were not excluded on the basis of previous or current use of antibiotics or analgesics. Patients were excluded if periodontal probing depths were greater than 4.0 mm on the experimental or control tooth or if bleeding on probing was detected. Patients were also excluded if undergoing orthodontic treatment.
GCF was collected from the experimental tooth and a healthy contralateral control tooth in each subject immediately before treatment. Contralateral teeth were examined clinically and radiographically, and cold testing was performed to ensure the tooth had normal pulp and periradicular tissues. One investigator (B.B.) collected all samples.
Before sample collection, the tooth was washed gently with water, dried, and isolated with cotton rolls. A saliva ejector was also used to prevent saliva contamination. Filter paper strips (Periopaper; Oraflow, Plainview, NY) were then inserted 1–2 mm into the gingival crevice of each tooth or until mild resistance was sensed. The strips were removed from the gingival sulcus after 30 seconds (Fig. 1). Two additional samples were collected from the same tooth at 5-minute intervals. Samples were rejected if contaminated by blood or saliva. A total of 6 strips were collected from each tooth. After removal from the gingival sulcus, all the strips were immediately placed in a microcentrifuge tube containing ice-cold 1X phosphate-buffered saline solution with 0.1 mmol/L phenylmethylsulfonyl fluoride. All patients were treated by endodontic residents according to established standard procedures for nonsurgical or surgical RCT. Patients were referred back to their general dentists for final restoration and were scheduled for a 6-month follow-up appointment in the Postgraduate Endodontics Clinic for assessment of healing and collection of GCF.
Figure 1.
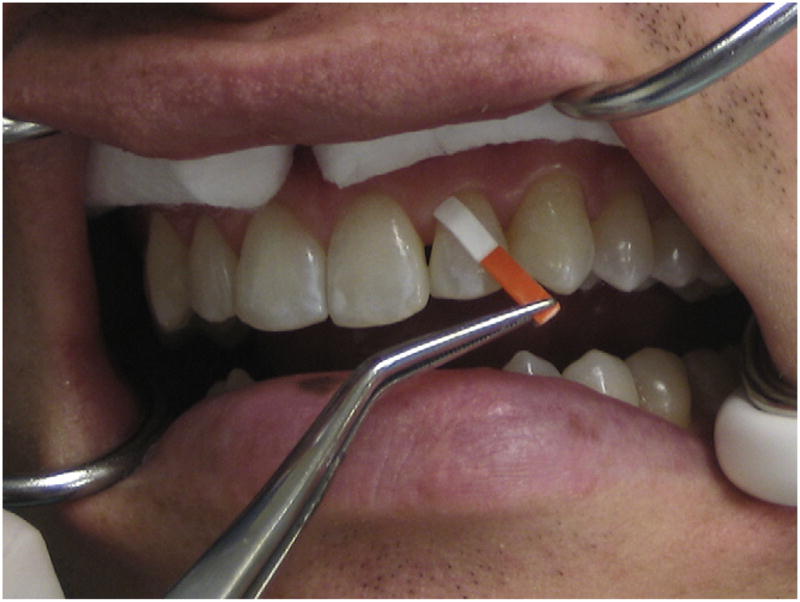
Filter paper strips were inserted 1–2 mm into the gingival crevice of each tooth for 30 seconds to collect the GCF sample. (This figure is available in color online at www.aae.org/joe/.)
The protein concentration of each sample was determined by the Bradford method (Bio-Rad protein assay; Bio-Rad, Hercules, CA) at 4°C to avoid protein degradation. Bovine serum albumin was used as the standard. After dilution of samples to equalize the protein content of each pair of control and experimental teeth, indirect enzyme-linked immunosorbent assay (ELISA) was used to detect and quantify the presence of IL-1β and DSP. All samples were assayed in duplicate. Primary antibody dilutions in this assay were 1:200 for IL-1β (Santa Cruz Biotech Co, Santa Cruz, CA) and 1:2000 for DSP (Dr Anne George, Chicago, IL). The secondary anti-rabbit immunoglobulin G antibody was used at a 1:7000 dilution (Sigma-Aldrich, St Louis, MO). The optical density was measured at 405 nm with a microtiter plate reader (Biotek, Winooski, VT).
An independent samples t test was used to compare the protein concentration and the levels of IL-1β and DSP in the GCF of experimental and control groups (SPSS, Chicago, IL). The significance level was set at P <.05.
Results
Forty diseased and 40 control teeth were tested (37 patients, 24 female and 13 male). The Bradford method for protein concentration showed an average of 66.52 ± 50.96 μg/μL in the diseased group and 36.69 ± 34.05 μg/μL in the control group. There was a statistically significant difference between the 2 groups (t = 3.08; P = .003) (Table 1). The mean absorbance of IL-1β in the GCF in the diseased group was 0.18 ± 0.06, which was not statistically different compared with the control group, 0.16 ± 0.05 (t = 1.62; P = 0.15) (Table 2). The mean absorbance of DSP was 0.34 ± 0.11 in the diseased group and 0.30 ± 0.09 in the control group. There was no statistically significant difference between groups (t = 1.78; P = .09) (Table 3).
TABLE 1.
Protein Concentration (μg/μL) in the Control and Disease Groups
| Group | N | Mean | Standard deviation | P value |
|---|---|---|---|---|
| Disease group | 40 | 66.52 | 50.96 | .003 |
| Control group | 40 | 36.69 | 34.05 |
TABLE 2.
IL-1β Absorbance in Control and Disease Groups
| Group | N | Mean | Standard deviation | P value |
|---|---|---|---|---|
| Disease group | 40 | 0.18 | 0.06 | .150 |
| Control group | 40 | 0.16 | 0.05 |
TABLE 3.
DSP Absorbance in Control and Disease Groups
| Group | N | Mean | Standard deviation | P value |
|---|---|---|---|---|
| Disease group | 40 | 0.34 | 0.11 | .086 |
| Control group | 40 | 0.30 | 0.09 |
Discussion
The outcome of RCT is influenced by a number of factors. One of the most important determinants of success is the status of the pulp and periapical tissues before treatment (10). Periapical radiographs are the most commonly used tool for evaluation of healing after RCT. However, the limitations associated with traditional 2-dimensional radiographic imaging to detect periapical pathosis are well-known (18, 23, 24). In addition, even though the majority of periapical lesions will show radiographic evidence of healing 1 year after treatment (25–27), healing progresses in a linear manner, and 3–4 years might be required to truly evaluate healing (28, 29). A noninvasive tool to measure the presence of active periapical inflammation could be a useful adjunct to radiographic evaluation, particularly in cases in which the radiographic interpretation is uncertain. Knowing the status of the immune response and not its consequences could be important in cases with apparently slow healing lesions.
The use of GCF as a diagnostic aid in periodontal disease is not a new concept (30, 31). However, use of GCF as a potential tool for diagnosis of periapical lesions of endodontic origin is a relatively novel concept. Belmar et al (32) found significantly elevated levels of matrix metalloproteinases (MMP-9 and MMP-2) in the GCF of teeth with periapical lesions. Although the study by Belmar et al was not available as a reference when the current study was designed and conducted, the methods were similar. MMPs in GCF might emerge as useful biological markers for monitoring apical periodontitis (32). Other investigators have found elevated levels of inflammatory markers in the GCF of teeth with a clinical diagnosis of irreversible pulpitis (21, 22). Specific organic matrix proteins and cytokines (osteopontin, osteoprotegerin, and receptor activator for nuclear factor kappa B ligand) have been found in higher levels in the GCF of teeth with root resorption as a result of orthodontic movement (33).
The results of this study demonstrated a higher level of IL-1β and DSP in the diseased group compared with the control group, but the results did not reach statistical significance. One possible explanation for this finding could be the dynamics of the development of apical periodontitis. This disease process is characterized by 2 distinct phases, an active bone resorption phase and a chronic phase with little lesion expansion (34). It is possible that IL-1β and DSP levels are elevated only during the active phase of the disease, and if this is the case, IL-1β and DSP might not be suitable markers for apical periodontitis.
Samples were prepared and diluted for the ELISA test in a way that equalized the amount of total protein in control and disease samples. This step was necessary because IL-1β and DSP are not exclusively seen in cases of apical periodontitis. The same markers were detected in patients with periodontal disease and root resorption. It was important to use a contralateral endodontically healthy tooth as a control in each patient to rule out false positives as a result of other inflammatory conditions.
Patients taking antibiotics and anti-inflammatory drugs were included in this study. The rationale for this decision was that many patients presenting for endodontic procedures are taking one or both of these types of medication. Even though anti-inflammatory drugs could decrease the amount of inflammatory mediators such as interleukin, these patients were included in the study.
Karapanou et al (22) showed an increased level of IL-8 (CXCL8) in the GCF of patients with irreversibly inflamed pulps compared with healthy contralateral teeth. An interesting finding in this study was that if samples were collected after the diseased tooth had received local anesthesia, the levels of CXCL8 dropped to levels similar to those found on healthy teeth, which shows the influence of anesthesia on the levels of CXCL8. We did not take the time of anesthesia into consideration in our study. Even though most of the samples were collected before anesthesia, some patients received anesthesia before collection, which might have influenced the results. Controlling for the influence of local anesthesia and concurrent use of antibiotics or anti-inflammatory drugs are relevant considerations for future research.
The presence of a significantly higher concentration of nonspecific protein in the GCF of diseased teeth compared with control teeth was an interesting finding and suggests a potential biochemical marker for periapical disease. Although the current study was unable to demonstrate a significant difference in IL-1β and DSP levels between diseased and control teeth, future studies might identify other biochemical markers for active apical periodontitis.
Acknowledgments
This research was funded in part by a grant from the American Association of Endodontists Foundation.
References
- 1.Barkhordar RA, Desouza YG. Human T-lymphocyte subpopulations in periapical lesions. Oral Surg Oral Med Oral Pathol. 1988;65:763–6. doi: 10.1016/0030-4220(88)90026-6. [DOI] [PubMed] [Google Scholar]
- 2.Metzger Z. Macrophages in periapical lesions. Endod Dent Traumatol. 2000;16:1–8. doi: 10.1034/j.1600-9657.2000.016001001.x. [DOI] [PubMed] [Google Scholar]
- 3.Dinarello CA. Biology of interleukin 1. Faseb J. 1988;2:108–15. [PubMed] [Google Scholar]
- 4.Heath JK, Saklatvala J, Meikle MC, Atkinson SJ, Reynolds JJ. Pig interleukin 1 (catabolin) is a potent stimulator of bone resorption in vitro. Calcif Tissue Int. 1985;37:95–7. doi: 10.1007/BF02557686. [DOI] [PubMed] [Google Scholar]
- 5.Wang CY, Stashenko P. Characterization of bone-resorbing activity in human periapical lesions. J Endod. 1993;19:107–11. doi: 10.1016/S0099-2399(06)80503-0. [DOI] [PubMed] [Google Scholar]
- 6.Barkhordar RA, Hussain MZ, Hayashi C. Detection of interleukin-1 beta in human periapical lesions. Oral Surg Oral Med Oral Pathol. 1992;73:334–6. doi: 10.1016/0030-4220(92)90131-9. [DOI] [PubMed] [Google Scholar]
- 7.Malueg LA, Wilcox LR, Johnson W. Examination of external apical root resorption with scanning electron microscopy. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996;82:89–93. doi: 10.1016/s1079-2104(96)80383-0. [DOI] [PubMed] [Google Scholar]
- 8.Balducci L, Ramachandran A, Hao J, Narayanan K, Evans C, George A. Biological markers for evaluation of root resorption. Arch Oral Biol. 2007;52:203–8. doi: 10.1016/j.archoralbio.2006.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qin C, Brunn JC, Cadena E, et al. The expression of dentin sialophosphoprotein gene in bone. J Dent Res. 2002;81:392–4. doi: 10.1177/154405910208100607. [DOI] [PubMed] [Google Scholar]
- 10.Friedman S, Abitbol S, Lawrence HP. Treatment outcome in endodontics: the Toronto Study—phase 1: initial treatment. J Endod. 2003;29:787–93. doi: 10.1097/00004770-200312000-00001. [DOI] [PubMed] [Google Scholar]
- 11.Pekruhn RB. The incidence of failure following single-visit endodontic therapy. J Endod. 1986;12:68–72. doi: 10.1016/S0099-2399(86)80131-5. [DOI] [PubMed] [Google Scholar]
- 12.Bender IB, Seltzer S. Roentgenographic and direct observation of experimental lesions in bone: I—1961. J Endod. 2003;29:702–6. doi: 10.1097/00004770-200311000-00005. discussion 701. [DOI] [PubMed] [Google Scholar]
- 13.Bender IB, Seltzer S. Roentgenographic and direct observation of experimental lesions in bone: II—1961. J Endod. 2003;29:707–12. doi: 10.1097/00004770-200311000-00006. discussion 701. [DOI] [PubMed] [Google Scholar]
- 14.Estrela C, Bueno MR, Leles CR, Azevedo B, Azevedo JR. Accuracy of cone beam computed tomography and panoramic and periapical radiography for detection of apical periodontitis. J Endod. 2008;34:273–9. doi: 10.1016/j.joen.2007.11.023. [DOI] [PubMed] [Google Scholar]
- 15.Estrela C, Bueno MR, Azevedo BC, Azevedo JR, Pecora JD. A new periapical index based on cone beam computed tomography. J Endod. 2008;34:1325–31. doi: 10.1016/j.joen.2008.08.013. [DOI] [PubMed] [Google Scholar]
- 16.Estrela C, Bueno MR, De Alencar AH, et al. Method to evaluate inflammatory root resorption by using cone beam computed tomography. J Endod. 2009;35:1491–7. doi: 10.1016/j.joen.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 17.Patel S. New dimensions in endodontic imaging: part 2—cone beam computed tomography. Int Endod J. 2009;42:463–75. doi: 10.1111/j.1365-2591.2008.01531.x. [DOI] [PubMed] [Google Scholar]
- 18.de Paula-Silva FW, Wu MK, Leonardo MR, da Silva LA, Wesselink PR. Accuracy of periapical radiography and cone-beam computed tomography scans in diagnosing apical periodontitis using histopathological findings as a gold standard. J Endod. 2009;35:1009–12. doi: 10.1016/j.joen.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 19.Cotton TP, Geisler TM, Holden DT, Schwartz SA, Schindler WG. Endodontic applications of cone-beam volumetric tomography. J Endod. 2007;33:1121–32. doi: 10.1016/j.joen.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 20.Linden GJ, McKinnell J, Shaw C, Lundy FT. Substance P and neurokinin A in gingival crevicular fluid in periodontal health and disease. J Clin Periodontol. 1997;24:799–803. doi: 10.1111/j.1600-051x.1997.tb01192.x. [DOI] [PubMed] [Google Scholar]
- 21.Awawdeh L, Lundy FT, Shaw C, Lamey PJ, Linden GJ, Kennedy JG. Quantitative analysis of substance P, neurokinin A and calcitonin gene-related peptide in pulp tissue from painful and healthy human teeth. Int Endod J. 2002;35:30–6. doi: 10.1046/j.1365-2591.2002.00451.x. [DOI] [PubMed] [Google Scholar]
- 22.Karapanou V, Kempuraj D, Theoharides TC. Interleukin-8 is increased in gingival crevicular fluid from patients with acute pulpitis. J Endod. 2008;34:148–51. doi: 10.1016/j.joen.2007.10.022. [DOI] [PubMed] [Google Scholar]
- 23.Goldman M, Pearson AH, Darzenta N. Endodontic success: who’s reading the radiograph? Oral Surg Oral Med Oral Pathol. 1972;33:432–7. doi: 10.1016/0030-4220(72)90473-2. [DOI] [PubMed] [Google Scholar]
- 24.Patel S, Dawood A, Whaites E, Pitt Ford T. New dimensions in endodontic imaging: part 1—conventional and alternative radiographic systems. Int Endod J. 2009;42:447–62. doi: 10.1111/j.1365-2591.2008.01530.x. [DOI] [PubMed] [Google Scholar]
- 25.Trope M, Delano EO, Orstavik D. Endodontic treatment of teeth with apical periodontitis: single vs multivisit treatment. J Endod. 1999;25:345–50. doi: 10.1016/S0099-2399(06)81169-6. [DOI] [PubMed] [Google Scholar]
- 26.Waltimo T, Trope M, Haapasalo M, Orstavik D. Clinical efficacy of treatment procedures in endodontic infection control and one year follow-up of periapical healing. J Endod. 2005;31:863–6. doi: 10.1097/01.don.0000164856.27920.85. [DOI] [PubMed] [Google Scholar]
- 27.Penesis VA, Fitzgerald PI, Fayad MI, Wenckus CS, BeGole EA, Johnson BR. Outcome of one-visit and two-visit endodontic treatment of necrotic teeth with apical periodontitis: a randomized controlled trial with one-year evaluation. J Endod. 2008;34:251–7. doi: 10.1016/j.joen.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 28.Peters LB, Wesselink PR. Periapical healing of endodontically treated teeth in one and two visits obturated in the presence or absence of detectable microorganisms. Int Endod J. 2002;35:660–7. doi: 10.1046/j.1365-2591.2002.00541.x. [DOI] [PubMed] [Google Scholar]
- 29.Weiger R, Axmann-Krcmar D, Lost C. Prognosis of conventional root canal treatment reconsidered. Endod Dent Traumatol. 1998;14:1–9. doi: 10.1111/j.1600-9657.1998.tb00801.x. [DOI] [PubMed] [Google Scholar]
- 30.Golub LM, Kleinberg I. Gingival crevicular fluid: a new diagnostic aid in managing the periodontal patient. Oral Sci Rev. 1976:49–61. [PMC free article] [PubMed] [Google Scholar]
- 31.Lamster IB. Evaluation of components of gingival crevicular fluid as diagnostic tests. Ann Periodontol. 1997;2:123–37. doi: 10.1902/annals.1997.2.1.123. [DOI] [PubMed] [Google Scholar]
- 32.Belmar MJ, Pabst C, Martinez B, Hernandez M. Gelatinolytic activity in gingival crevicular fluid from teeth with periapical lesions. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;105:801–6. doi: 10.1016/j.tripleo.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 33.George A, Evans CA. Detection of root resorption using dentin and bone markers. Orthod Craniofac Res. 2009;12:229–35. doi: 10.1111/j.1601-6343.2009.01457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stashenko P, Yu SM, Wang CY. Kinetics of immune cell and bone resorptive responses to endodontic infections. J Endod. 1992;18:422–6. doi: 10.1016/S0099-2399(06)80841-1. [DOI] [PubMed] [Google Scholar]


