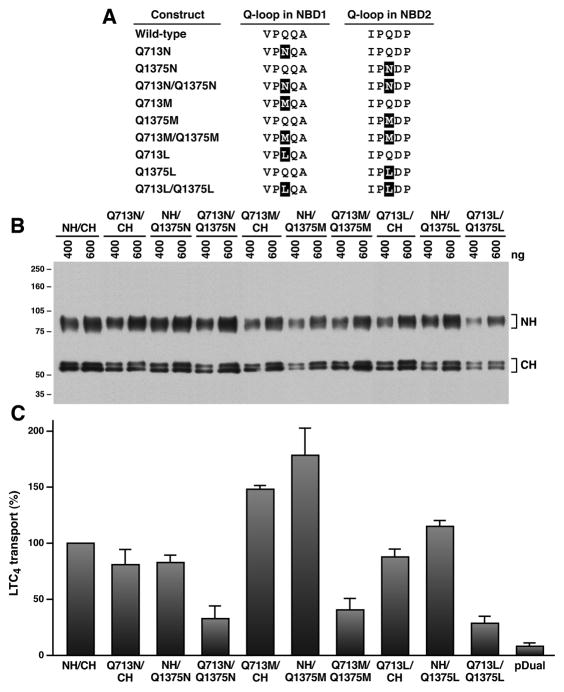
Figure 1. Substitution of Q713 in Q-loop of NBD1 or Q1375 in Q-loop of NBD2 with an amino acid that eliminates the amide group did not have a significant effect on the Mg·ATP-dependent LTC4 transport.
A. Linear amino acid sequences around the glutamine residue in Q-loop. The highlighted amino acids indicate the mutated residues. B. Expression of the N-half and C-half of MRP1 mutants in insect cells. Membrane vesicles were prepared from Sf21 cells infected with viral particles harboring pDual/NH/CH of MRP1 cDNA. The amounts (ng) of membrane proteins loaded in the gel are indicated on top of the gel. Molecular weight markers are indicated on the left. NH and CH on the right indicated the N-half and C-half of MRP1 protein. C. Relative ATP-dependent LTC4 transport activities of the wt and mutated MRP1s. ATP-dependent LTC4 transport was performed according to the methods described in Materials and methods. Since the amounts of MRP1 proteins determined in Figure 1B were different, the amounts of membrane vesicles used in these experiments were adjusted, based on the ratio of MRP1 protein in membrane vesicles listed in Table S2, to have a similar amount of MRP1 protein by adding varying amounts of membrane vesicles prepared from insect cells infected with viral particles harboring pDual expression vector. For example, 1.56 μg of wt MRP1 + 1.44 μg of pDual and 3.00 μg of Q1375M were used to determine the ATP-dependent LTC4 transport activities of wt MRP1 and Q1375M-mutated MRP1. The data, considering the transport activity catalyzed by wt MRP1 as 100%, are presented as means ± standard deviation of three independent experiments.