Figure 2.
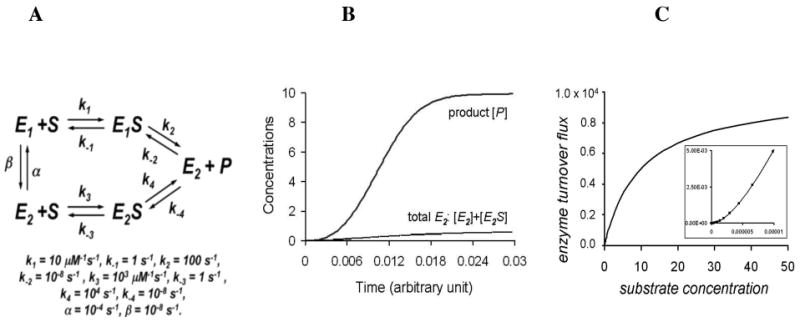
A. A variation of the general fluctuting enzyme kinetic model with two conformational substates E1 and E2, which yield the same product from a single substrate with different kinetic parameters. In this example, we specifically assume that E2 is a better catalyst than E1. The parameters used for the simulations are shown. In particular, we have assumed that the enzyme is inevitably in the state E2 after accomplishing a catalysis step. B. The concentration of product (P) or all forms of E2 ([E2] + [E2S]) as a function of time. There is marked hysteresis (lag) in the formation of product as the population of enzyme states shifts toward a greater fraction of E2 with increasing turnover. C. The rate of product formation vs. [S]. The inset is a close up of the low [S] range, and demonstrates the sigmoidal dependence of rate on [S].
