Figure 4.
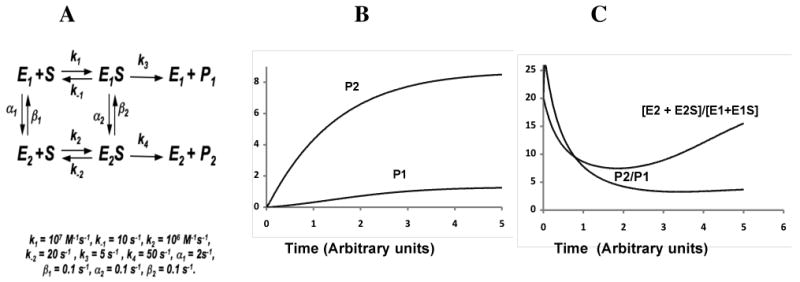
A. Minimal kinetic scheme for two enzyme conformations generating a multiple products from a single substrate. In the simulations E2 is a more efficient catalyst than E1. B. Rate of formation of P1 and P2. Note the hysteresis in the formation of P1. C. Ratios of all enzyme forms E2 vs. E1 ([E2+E2S]/[E1+E1S]) or the product ratio (P2/P1) vs. time. The enzyme forms ‘adapt’ from the initial preference for E2 in the absence of substrate to a more ‘balanced’ ratio of all E2 forms/all E1 forms, and increases again as substrate is depleted.
