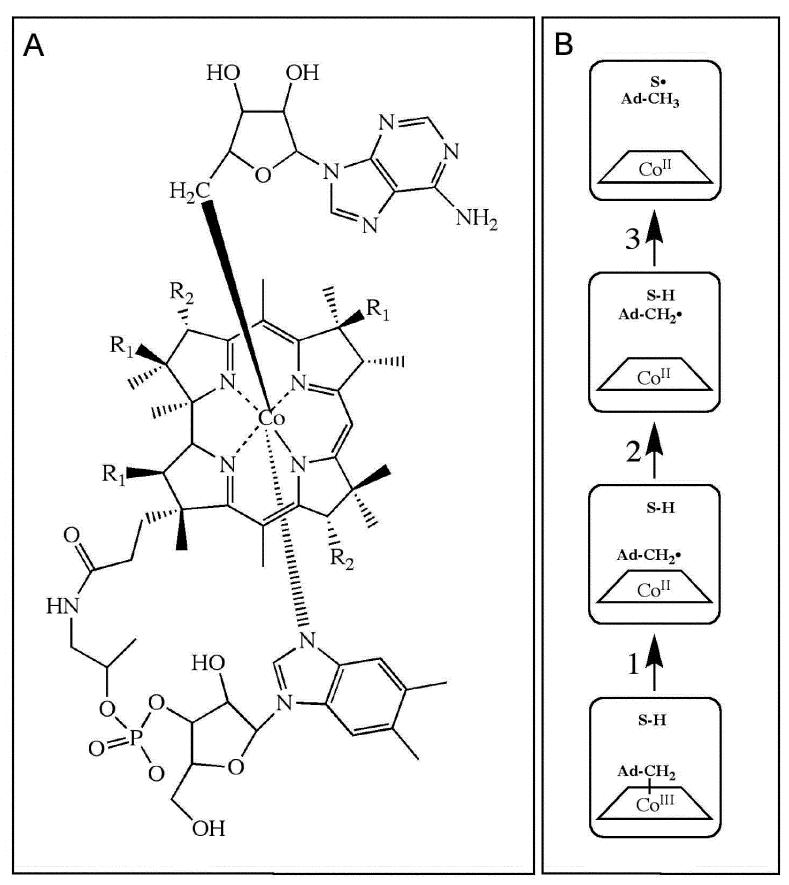
Figure 1.
Depiction of the structure of adenosylcobalamin and canonical states and steps in the radical pair creation and separation reaction sequence in EAL. (A) Structure of adenosylcobalamin. The β-axial ligand is 5′-deoxyadenosyl. The dimethylbenzimidazole α-axial ligand of the coenzyme remains coordinated when the coenzyme is bound to EAL.63,64 R1 and R2 represent acetamide and propionamide side chains, respectively. (B) Canonical states and steps in the native radical pair creation and separation reaction sequence in EAL.1,14 The steps are as follows: (1) Cobalt-carbon bond cleavage, (2) radical pair migration, and (3) hydrogen atom transfer. Substrate-derived species are designated S-H (bound substrate) and S• (substrate radical). The 5′-deoxyadenosyl β-axial ligand is represented as Ad-CH2- in the intact coenzyme, and as Ad-CH2• (5′-deoxyadenosyl radical) or Ad-CH3 (5′-deoxyadenosine) following cobalt-carbon bond cleavage. The cobalt ion and its formal oxidation states are depicted, but the corrin ring and the dimethylbenzimidazole α-axial ligand of the coenzyme are not shown for clarity.