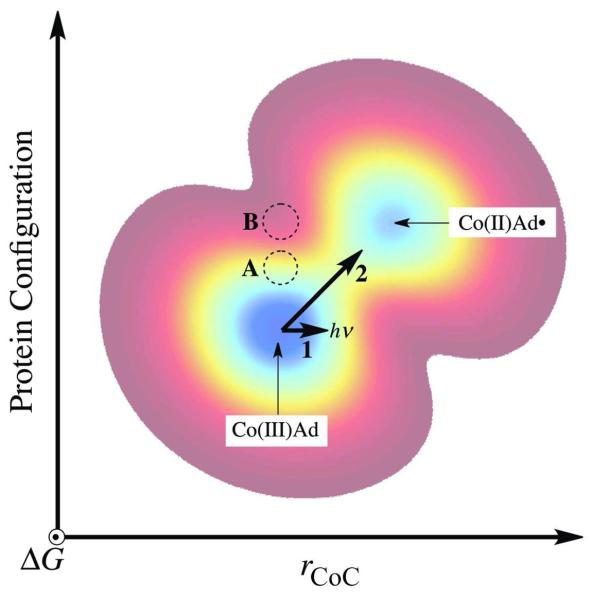
Figure 9.
Two-dimensional contour representation of the ground state free energy surface for the ternary complex and radical pair formation and separation in EAL as a function of chemical (Co-C bond cleavage) and protein configuration coordinates. The two minima represent the ternary complex [Co(III)Ad] and the cob(II)alamin-5′-deoxyadenosyl radical pair [Co(II)Ad•] states. The one-dimensional trajectory for photolysis (Path 1) is represented by a horizontal bold arrow. The trajectory for the native thermal Co-C bond cleavage is represented by the diagonal bold arrow (Path 2). A representative position of the free energy minimum for the holo-EAL state is given by the region marked “A.” The region, “B,” marks a representative position for the free energy minimum of a protein state, that might be obtained by a simple substrate binding-induced switch of the holo-EAL protein configuration to one that favors prompt stabilization of the photoproduct radical pair.