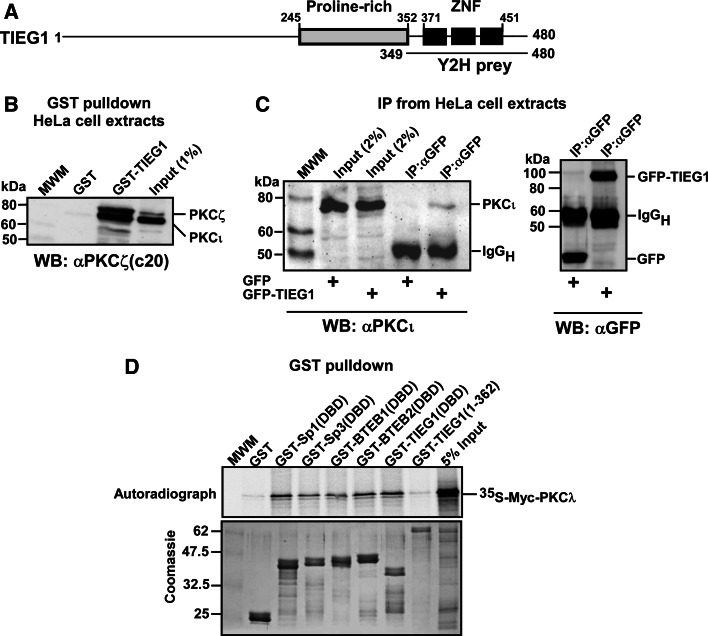
Fig. 1.
PKCs ζ and ι interact with the KLF family zinc finger domain in vitro and in HeLa cells. a Schematic representation of the domain structure of TIEG1. The three zinc finger motifs constituting the DNA-binding domain are represented by filled boxes. The TIEG1 region isolated in the yeast two-hybrid screen is indicated by the solid line below the schematic. b Whole-cell extracts from HeLa cells were incubated with equal amounts of bacterially expressed GST or GST-TIEG1 immobilized on glutathione-sepharose beads. The presence of aPKCs in the pulled-down proteins were examined by immunoblotting using αPKCζ(C20) antibody that recognizes both PKCζ and PKCι. c U2OS cells were transfected with either GFP or GFP-TIEG1. The cells were lysed 24 h post transfection, and immunoprecipitations were performed using anti-GFP antibody. Western blots of the immunoprecipitates and of the cell extract were revealed using monoclonal anti-PKCλ antibody (left panel) and immunoprecipitated GFP or GFP-TIEG1 proteins were visualized using polyclonal anti-GFP antibody (right panel). d Myc-tagged PKCλ was in vitro translated in the presence of [35S] methionine and incubated with equal amounts of either glutathione-sepharose beads coupled GST or GST-tagged KLF family proteins. The pulled-down proteins together with 5% of the input were subjected to detection by autoradiography (upper panel). The levels of GST or GST-tagged proteins used in the GST pulldown assays were examined by Coomassie brilliant blue staining (lower panel). The data shown in b, c, and d correspond to a representative experiment out of two performed