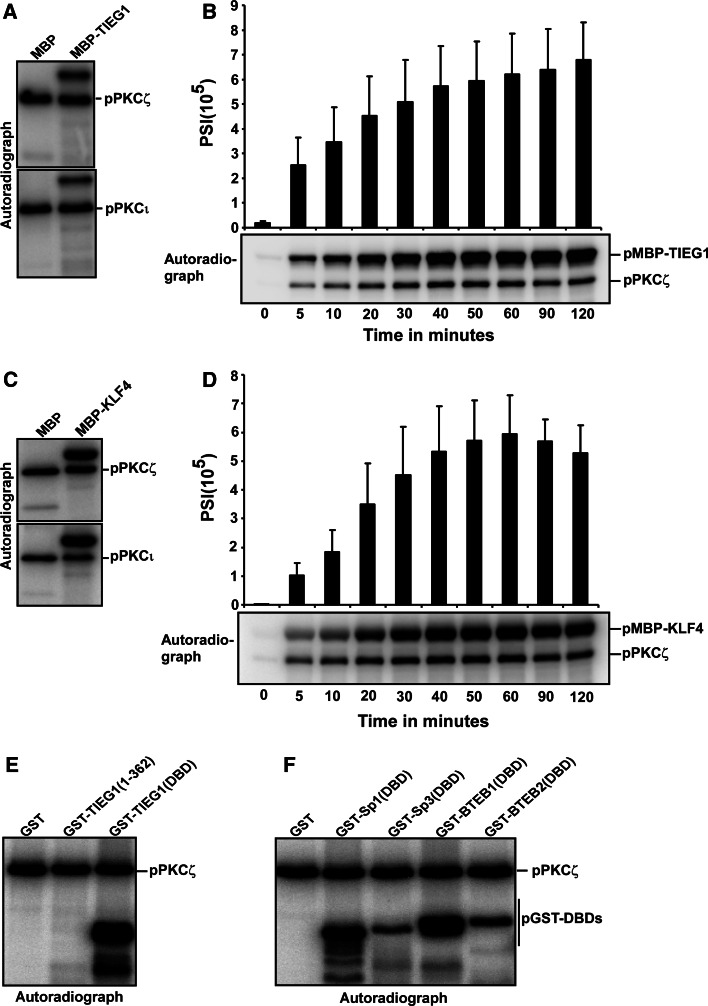
Fig. 2.
The KLF family proteins are phosphorylated by the atypical PKCs ζ and ι. a, c Both PKCζ and PKCι phosphorylate TIEG1 (a) and KLF4 (c) in vitro. The phosphorylation assays were performed for 20 min at 30°C in 30-μl reaction volume containing MBP, and MBP-TIEG1 or MBP-KLF4 as substrate, 50 ng recombinant active PKCζ (upper panels) or PKCι (lower panels) 60 μM unlabeled ATP and 2 μCi [32P]-ATP. The reactions were analyzed by autoradiography. b, d The phosphorylation of TIEG1 (b) and KLF4 (d) by PKCζ is linear with time. The upper panels are bar graphs showing the quantification of the phosphorylation levels of TIEG1 and KLF4 over the indicated time points, respectively, and the lower panels are the corresponding autoradiographs. e, f The DNA-binding domains of KLF family proteins are targets for PKCζ-mediated phosphorylation. GST, GST-TIEG1(1–362) and GST-TIEG1(DBD) (e), and GST, GST-tagged DBDs of KLF family proteins (f) were subjected to in vitro phosphorylation and subsequent analysis by autoradiography. The data in a, c, e and f are representative of three independent experiments. The data in b and f are shown as the mean (± SD) of four independent experiments