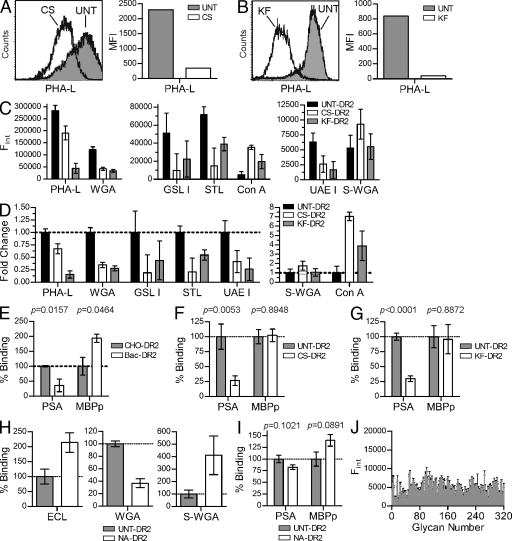
Figure 4.
Both native N-glycans and the HLA-DR2 protein backbone are required for PSA binding. (A and B) Flow cytometry analysis of CS- and KF-treated CHO cells were compared with UNT cells using a lectin sensitive to the presence of native complex N-glycans. Representative histograms are shown. (C and D) HLA-DR2 expressed by UNT, CS-treated, and KF-treated CHO cells was immobilized and probed using a panel of FITC-conjugated lectins to detect changes in MHCII glycosylation. The raw data showing relative concentrations of the target glycan structure (D) and fold change (E) of each lectin is shown. n = 9 per data point. (E) Quantitative binding experiments using PSA or MBPp with purified HLA-DR2 expressed in bacteria (Bac-DR2; nonglycosylated) or DR2 expressed in CHO cells (CHO-DR2; fully glycosylated). n = 4 per data point. (F and G) MBPp and PSA binding to DR2 lacking native N-glycans expressed in CHO cells grown in CS-supplemented (n = 9; F) or KF-supplemented media (n = 18; G) compared with CHO-DR2 expressed in the absence of inhibitor. (H) Lectin ELISA of neuraminidase-digested CHO-DR2. (I) Native CHO-DR2 enzymatically trimmed with α(2→3,6,8,9)-neuraminidase (NA-DR2) binding to PSA or MBPp compared with untreated CHO-DR2. n = 21. (J) Alexa Fluor 488–conjugated PSA was used as ligand in a glycan-binding array containing 320 glycans (Table S1) at the Consortium for Functional Glycomics. Positive signal is typically above 20,000 U. n = 4 per glycan. All bar graphs show the mean ± SEM.