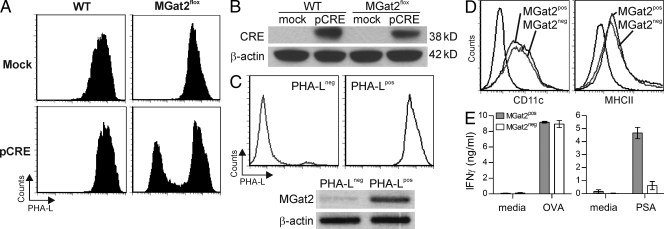
Figure 7.
Deletion of MGat2 results in deficient PSA, but not OVA-mediated T cell activation. (A) BM cells were harvested from MGat2wt (WT) or MGat2flox animals, electroporated with a CRE expression construct (pCRE) or mock electroporated without DNA, and cultured with GM-CSF for DC differentiation. Cells were stained with PHA-L to determine the effect of MGat2 excision. Representative histograms are shown. (B) Mock and pCRE electroporated BM cells were extracted and lysates were analyzed by anti-CRE Western blot using β-actin as a loading control. Representative blot shown. (C) PHA-L− pCRE electroporated MGat2flox BMDCs were isolated from PHA-L+ cells by negative selection using PHA-L and magnetic beads and compared with PHA-L+ pCRE-electroporated WT cells. Flow cytometry was used to measure the purity and glycophenotype of the populations, whereas RT-PCR on mRNA isolated from the PHA-Lpos and PHA-Lneg BMDCs was performed to demonstrate the excision of MGat2 in PHA-Lneg BMDCs (MGat2neg) but not PHA-Lpos BMDCs (MGat2pos). Representative histograms and gel shown. (D) Flow cytometry was used to measure BMDC differentiation (CD11c) and MHCII expression (MHCII) in both PHA-Lpos and PHA-Lneg BMDC populations. Representative histograms shown. (E) MGat2pos and MGat2neg BMDCs were cultured with either OT-II CD4+ T cells and OVA (50 µg/ml) or WT CD4+ T cells and PSA (50 µg/ml). T cell activation was determined by IFN-γ ELISA of the culture supernatants. (n = 3 independent experiments).