Figure 1. Multifactorial nature, pivotal role, and mechanism of AD neurofibrillary degeneration.
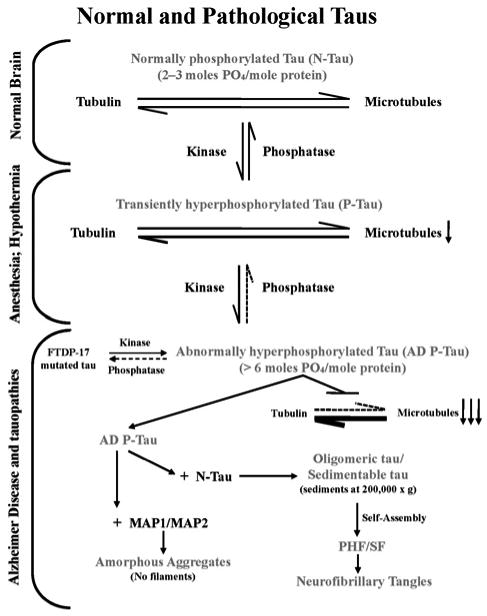
Neurofibrillary degeneration of the AD type is a product of multiple aetiological factors. Independent of the aetiology, neurofibrillary degeneration in AD and tauopathies involves abnormal hyperphosphorylation of tau and its occurrence in the neocortex is associated with dementia; neurofibrillary degeneration in the brainstem in progressive supranuclear palsy is associated with motor impairment. A cause of hyperphosphorylation of tau is a decrease in the activity of PP2A, a major tau phosphatase. Unlike normal tau, AD P-tau, instead of interacting with tubulin and promoting its assembly into microtubules, sequesters normal tau, MAP1 and MAP2, forming 200 000 g sedimentable oligomeric tau. The sequestration of normal MAPs disrupts microtubules and compromises axonal transport and consequently retrograde degeneration and dementia. The AD P-tau polymerizes into tangles of PHFs/SFs, which, unlike the cytosolic/oligomeric AD P-tau, interacts neither with tubulin nor with normal MAPs, but eventually obstructs further the microtubule network and axoplasmic flow as a space-occupying lesion.
