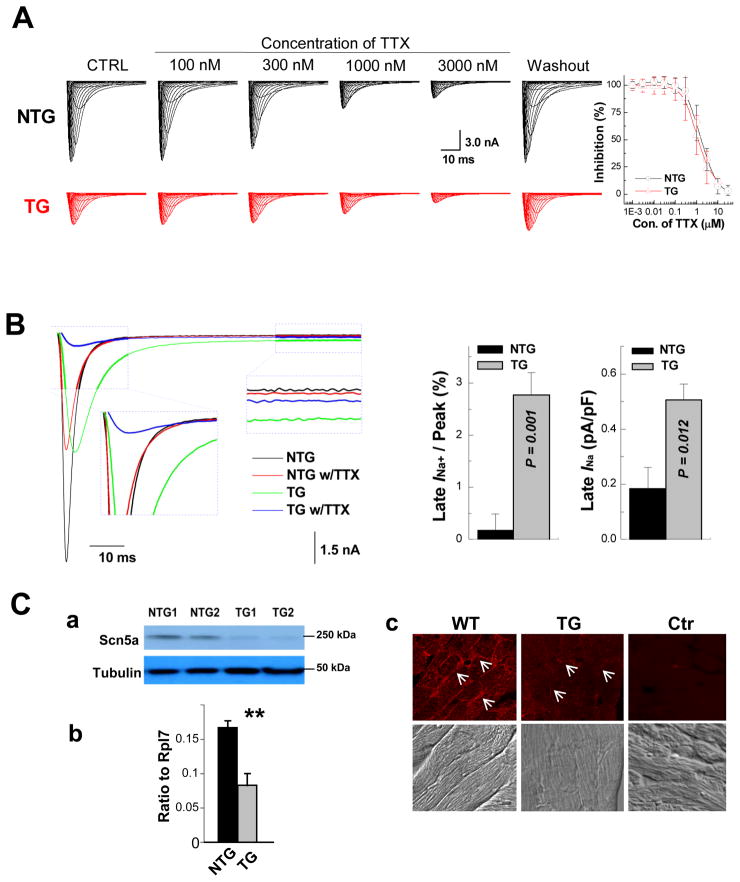
Figure 4.
Assessment of late INa. A, Ventricular cardiomyocytes from αMyHC-FKBP12 and wild-type mice express the TTX-resistant (cardiac-isoform) but not TTX-sensitive (neuronal-isoforms) of voltage-gated Na+ channels. (Left panels) Representative INa responses to increasing concentrations of extracellular TTX in TG and NTG ventricular cardiomyocytes. (Right panel) Plots of the percent inhibition of peak INa as a function of TTX concentration in transgenic (n=4) and non-transgenic (n=3) myocytes. Mean Hill coefficients for TTX-block of peak INa were 1.2 and 1.3 (P>0.05) for the NTG and TG cardiomyocytes, suggesting that a single TTX molecule blocks a single Na+ channel. Nanomolar concentrations of TTX that typically block neuronal-isoforms of voltage-gated Na+ channels had no detectable effect on peak INa in either cardiomyocyte-type. B, FKBP12 overexpression enhances a persistent Na+ current. Original traces of INa elicited from −100 mV to +30 mV for 400 ms before and after exposure to 3 μM TTX in the external solution. TTX-sensitive components were averaged over the last 100 ms of the depolarizing pulse and normalized to the cell capacitance. FKBP12 overexpression increased mean late INa density (P<0.05). C, Assessment of Nav1.5 expression and cellular localization. Western blot (a), qRT-PCR (b), and immunofluorescence and confocal imaging (c) analyses demonstrated a significant reduction of Nav1.5 in transgenic hearts. Confocal images were representative for three independent sets of experiment. White arrows (in c) denote anti-Nav1.5 immune reactivity in the outer surface membrane of cardiomyocytes. **P < 0.01.