Abstract
The cervical mucosa of women who are highly exposed to HIV-1, yet remain persistently seronegative (HEPS), presents a unique opportunity to study the dynamics of an immune compartment potentially capable of preventing HIV-1 infection. Herein, we provide a detailed characterization of the immunoglobulin repertoire of cervical and systemic B cells from one such HEPS individual from Nairobi, Kenya. Analysis was done on 512 VH sequences that were RT-PCR amplified from B cells in a paired sample from the cervix and peripheral blood. The VH3 and DH repertoire of class switched cervical B cells differs significantly from that of systemic B cells, indicating that the cervical environment affects local B-cell populations and hence VH gene expression. Six networks of clonally related, heavily mutated B cells were identified that spanned the systemic and cervical B-cell compartments. Analysis of somatic mutations suggests this is likely the result of systemic, class switched B cells homing to the cervical mucosa. Multiple networks of somatically mutated V-gene sequences, unique to the cervical mucosa, were also identified. This supports the notion that site specific responses occur and have unique regulation of tolerance and recruitment into local memory or blast B-cell compartments. We conclude that while the nature of the cervical environment shapes the local B cell repertoire, the infusion of post germinal center B cells to the human cervix is a common occurrence, and represents a means by which systemic immunization could provide the local antibodies necessary to prevent HIV-1 at the site of initial contact.
Key words: immunogenetics, HIV-1, B cells, cervical mucosa, somatic mutation, HIV resistance
Introduction
Despite almost 30 years of research, the human immunodeficiency virus (HIV-1) remains a significant health burden worldwide. The vaccine required to stop the epidemic will require an effective cell-mediated and humoral immune response;1 however, the foremost B-cell response is primarily directed against non-neutralizing epitopes and secondary responses consistently lag behind the mutating virus.2,3 Consequently, protection against HIV-1 will likely require the presence of protective mucosal antibodies at the site of first contact.4 Because over 80% of infections are acquired through heterosexual intercourse,5 elucidating the details of a protective mucosal antibody response against HIV-1 in the human cervix is of obvious importance. HIV-1 specific IgA and, to a lesser extent, IgG, have been isolated from cervico-vaginal secretions in some individuals who are highly exposed yet remain persistently seronegative to HIV-1 (HEPS).6–10 Other studies on the mucosal response against HIV-1 have produced inconsistent data regarding the contribution, specificities and breadth of neutralization of both IgA and IgG.11 Nevertheless, mucosal IgA has been declared a correlate of protection against HIV-1.10,12
While the nature of the mucosal antibody response against HIV-1 represents a considerable gap in knowledge of HIV-1 pathogenesis, the understanding of the relationship between the systemic and genital tract immune compartments is even more elementary. A member of the common mucosal immune system, the human cervix represents a unique mucosal compartment in that circulating plasma-derived immunoglobulin (Ig) makes up a substantial portion of total antibody levels in cervicovaginal secretions.13 Immunizations delivered systemically and to distant mucosal sites have produced vaccine-specific IgG and IgA at the genital tract.5,14–18 To what degree immunity in the genital tract is dependent on Ig transport from the serum, as shown in monkeys,4 or B-cell homing from the periphery, remains unclear. Considering the protective efficacy of mucosal Ig, defining the relationship between the systemic and genital humoral immune compartments at a molecular level is of high priority.
The ontogeny of B cells in different immune compartments can be investigated by examining their rearranged and expressed Ig variable heavy (VH) chain genes.19–21 The human immunoglobulin heavy chain complex is located primarily on chromosome 14,22 and consists of 123–129 VH,23–25 27 diversity (DH),26,27 9 junction (JH)28 and 11 constant (CH) gene segments.29,30 The VH gene segment can be grouped into seven families (VH 1–7) based on sequence homology among framework regions (FR) 1, 2 and 3 and complementarity determining regions (CDR) 1 and 2. While enormous diversity exists at the germline level, it is not clear what amount of this entire diversity is expressed in a given B cell compartment. The expressed VH repertoire is shaped further by the combinatorial and junctional joining of the V, D and J segments.31 Additional somatic diversity is generated by affinity maturation in which the initial V-D-J templates are subject to somatic hypermutation.32 Systemically, B-cell centrocytes with mutations improving antigenic affinity are positively selected and expanded, presumably in germinal centers.33–35 Despite this huge potential diversity, half of all germline VH genes expressed in adult peripheral B cells can be classified as one of the 22 functional VH3 genes.25 The biased expression of VH genes in peripheral B cells is determined primarily by preferential rearrangement during V-D-J recombination.36 However, the expression and affinity maturation of VH genes in mucosal B-cell compartments is understudied and it remains unclear how similar the ontogeny is among compartments. Our studies herein are directed at creating an initial understanding of the B cells and VH gene repertoire present locally in the human cervix.
Detailed analysis of B-cell circulation between the genital tract and peripheral blood immune compartments is needed to elucidate their respective roles in fighting HIV-1. While not an exhaustive list, this relationship can be studied in several ways: First, by examining the VH genes of individual B cells of a particular phenotype37; second, by conducting comprehensive deep sequencing of the VH transcriptome;38,39 third and most simply, by examining a specific subset of VH genes through the use of a limited set of oligonucleotide primers.40 In the present study, we examined the expression of a major VH subset in a paired sample of lymphocytes isolated from the cervix and peripheral blood of an HEPS individual. The expressed and rearranged VH3 family of Ig RNA were amplified and sequenced from each sample using a biased primer set, and provides evidence for the compartmentalization of the humoral immune system in the female genital tract. Furthermore, while analysis of VH genes from local B cells of the cervix cannot explicate the significance of plasma-derived Ig in the cervix, we identified six networks of clonally related, heavily mutated B cells spanning both immune compartments. Our characterization of the molecular details of this relationship contributes to our growing understanding of genital tract immunity.
Results
Cloning and sequencing.
Four unique mutations were observed in a total of nearly 15,000 sequenced base pairs of Cγ-1 from the 40 productive sequences from the peripheral blood sample. This is consistent with the estimated error rate of the proof reading Tgo DNA polymerase mix used, and served as our internal control for the rate of PCR mutations.48 The PCR error rate was estimated at 1 base substitution for every 10 VH sequences analyzed.
A total of 512 VH sequences were RT-PCR amplified and cloned from B cells in a paired CMC and PBMC sample. Non-productive sequences were ignored, and identical sequences of the same isotype and location were considered a single sequence. Applying these criteria, the 356 productive VH sequences isolated from the HEPS individual are summarized in Table 2. A significantly higher frequency of unique VH-DH-JH rearrangements were identified in the IgG (p = 0.002) and IgM (p = 0.046) PBMC sample relative to the CMC sample. Alternatively, the CMC IgA sample was significantly more diverse than the IgA PBMC sample in that 35 of the 38 productive sequences (92%) isolated from the cervix were identified as unique rearrangements, compared to 26 of 41 productive sequences (63%) from the peripheral blood (p = 0.003). This suggests a greater proportion of the local B-cell population in the cervix are IgA producing and is consistent with the predominance of IgA plasmablasts within the endo- and ecto-cervix (65–79%).49
Table 2.
Summary of the VH sequences derived from B cells of different isotypes in the cervix or peripheral blood
| Summary | IgA | IgG1 | IgG2 | IgG3 | IgM | |||||
| Cervix | Blood | Cervix | Blood | Cervix | Blood | Cervix | Blood | Cervix | Blood | |
| Sequences Analyzed | 48 | 48 | 48 | 48 | 48 | 48 | 48 | 48 | 64 | 64 |
| Uniquea and Productiveb | 38 | 41 | 30 | 40 | 26 | 35 | 22 | 28 | 40 | 56 |
| Unique V-D-J Rearrangementsc | 35 | 26 | 24 | 38 | 23 | 34 | 12 | 23 | 30 | 51 |
Identical sequences of the same isotype were considered a single sequence.
A sequence was considered unique if it did not share 100% homology with another clone of the same isotype, and productive if it contained no stop codons.
The number of different V-D-J combinations was determined considering only productive sequences and provides an indication of diversity when compared with the corresponding number of total unique productive sequences.
VH3 and DH gene usage in the cervix and peripheral blood.
Due to the highly conserved nature of FR-1, a small number of sequences from VH families 1, 5, 4b and 6 were amplified using the VH3 specific sense primer; however, no significant differences in their respective gene frequencies between the systemic and mucosal B-cell compartments were observed (data not shown).
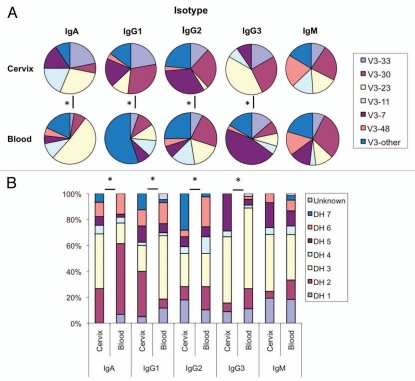
There are obvious differences in VH3 and DH gene representation in the cervix versus the blood at the time point evaluated (Fig. 1). Use of the family specific primers allowed for a direct comparison of the representation of the specific gene families listed in Figure 1A. Within isotype comparisons showed that B cells rearranged as the IgA and IgG isotype each have a different profile of VH3 gene segment use in the cervix compared to the peripheral blood (p ≤ 0.05). Only B cells of the IgM isotype did not have a different VH3 gene expression profile between the two populations (p ≤ 0.05). Despite the difficulties in assigning germline D genes given the high mutation rate, the DH gene expression profile within isotypes mirrored the above results in that DH usage in isotype switched B cells differed between the cervical and peripheral blood compartments (p ≤ 0.05) (Fig. 1B). No significant differences in JH gene usage between the two compartments were observed (data not shown).
Figure 1.
Nature of the cervical environment shapes local VH gene expression. (A) VH3 gene segment frequencies from B cells of each isotype isolated from the cervix or peripheral blood. V3-other is defined as any VH3 locus other than those defined above. (B) DH gene segment frequencies from B cells of each isotype isolated from the cervix or peripheral blood. Significant differences (*) in both VH and DH gene usage were observed between each of the isotype-matched samples with the notable exception of IgM using χ2 tests (p ≤ 0.05).
Frequencies of somatic mutation in the cervical mucosa and peripheral blood.
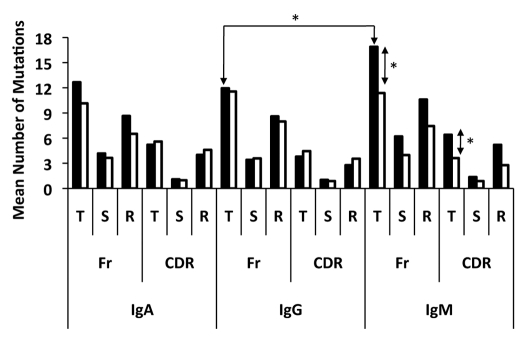
A detailed analysis of the somatic mutations in both immune compartments is presented in Table 3. Collectively, the frequency of somatic mutation among all isotypes was significantly higher in cervical B cells (6.5%) compared to systemic B cells (5.3%) (p ≤ 0.05). When considered individually each isotype tested had a higher frequency of mutation in the cervical mucosa than did the matched sample from peripheral blood, although this was not statistically significant (Table 3). IgG2 bearing B cells had the highest frequency of mutation of all the isotypes in both the cervix (8.5%) and peripheral blood (7.4%). Interestingly, the largest difference in the frequency of mutation between the cervix and peripheral blood was within the IgM isotype. The cervical IgM population displayed a significantly greater mean number of total mutations in both the CDR and FR regions when compared with the IgM population in the peripheral blood (p ≤ 0.05) (Fig. 2). Furthermore, isotype comparisons revealed that cervical VH genes rearranged as the IgM isotype had a higher mean number of total FR mutations than both IgA and pooled IgG, although only significant for IgG (p ≤ 0.05) (Fig. 2).
Table 3.
Mutation statistics for VH sequences B cells of each isotype isolated from the cervix or peripheral blood
| Isotype | Source | Mutation Frequency (%) | Mean Number of Mutations | R:S Value CDRs 1–2a | R:S Value FRs 1-3a | Mean P FRb | Mean P CDRc |
| IgA | Blood | 6.2 | 15.7 | 4.74 | 1.81 | 0.162 | 0.166 |
| Cervix | 6.7 | 17.8 | 3.70 | 2.07 | 0.238 | 0.286 | |
| IgG1 | Blood | 4.3 | 12.2 | 3.73 | 3.09 | 0.376 | 0.307 |
| Cervix | 5.3 | 12.8 | 2.72 | 1.91 | 0.314 | 0.344 | |
| IgG2 | Blood | 7.4 | 21.3 | 3.92 | 1.87 | 0.194 | 0.244 |
| Cervix | 8.5 | 19.8 | 3.45 | 3.09 | 0.301 | 0.358 | |
| IgG3 | Blood | 4.8 | 13.9 | 5.07 | 2.16 | 0.329 | 0.383 |
| Cervix | 5.3 | 14.9 | 1.84 | 2.61 | 0.412 | 0.581 | |
| IgM | Blood | 5.6 | 15.0 | 3.16 | 1.87 | 0.311 | 0.411 |
| Cervix | 7.5 | 23.3 | 3.82 | 1.71 | 0.236 | 0.291 |
Mean R:S values were calculated after pooling VH sequences according to isotype and location.
Individual P FR values were calculated for each sequence and the mean subsequently determined for each isotype. The P FR value is an estimation of the probability that the observed scarcity of R mutations in FRs 1–3 occurred by random mutation and not through antigen driven affinity maturation.46
Individual P CDR values were calculated for each sequence and the mean subsequently determined for each isotype. The P CDR value is an estimation of the probability that the observed excess of R mutations in CDRs 1,2 occurred by random mutation and not through antigen driven affinity maturation.46
Figure 2.
Distribution of somatic mutations in B cells isolated from the cervix (dark bars) and peripheral blood (light bars). Shown is the mean number of total (T), silent (S) and replacement (R) nucleotide mutations for each isotype. IgG1, IgG2 and IgG3 VH populations were pooled forming one IgG population. (*) is indicative of a signficicant difference (p ≤ 0.05) between the mean number of mutations indicated.
Evidence of additional antigen-driven affinity maturation in the peripheral blood compared to the cervical mucosa.
The numbers of R and S nucleotide mutations for individual VH sequences of each isotype were compared with the expected numbers of mutations in the most homologous germline gene given random mutation. Using the multinomial model described below, each VH sequence was given a p value describing the probability that the observed excess or scarcity of R mutations in the FR or CDRs respectively occurred by random mutation. The mean p values estimating selection in the FRs and CDRs for each isotype is presented in Table 3. B cells of the IgA and IgG3 isotype had a significantly lower mean p CDR value in the peripheral blood compartment when compared with the cervical mucosa (p ≤ 0.05), indicating that IgA and IgG3 switched B cells are under less selective pressure in cervical mucosa when compared with the isotype matched cells in the peripheral blood. Although statistically insignificant, this trend holds true for both IgG1 and IgG2. Interestingly, B cells of the IgM isotype resist this trend as IgM-VH sequences from the cervix showed a significantly lower mean p CDR value than in the peripheral blood (p ≤ 0.05) (Table 3). Although not significant, these trends hold true when examining mean p FR values.
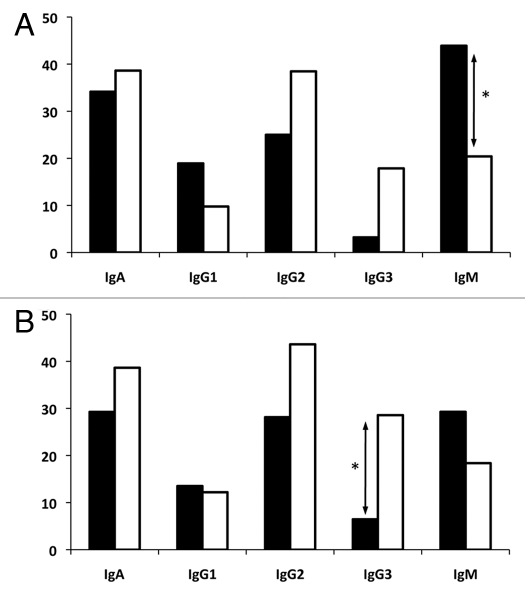
The antigenic selection index differed between peripheral blood and cervical B-cell VH genes. When the p value of each VH sequence was considered individually, the frequency of antigenic selection could be determined by calculating the number of VH sequences with a p CDR or p FR ≤0.05 (Fig. 3). A trend towards a higher inferred frequency of antigenic selection in the peripheral blood compartment relative to the cervical compartment was observed for B cells of the IgA, IgG2 and IgG3 isotypes, although only significant when examining the CDRs of IgG3 (p < 0.05) (Fig. 3B). The frequency of antigenic selection in the cervix was comparable with that of the blood for IgG1 B cells. Alternatively, and consistent with data presented in Table 3, IgM cervical B cells showed a significant increase in the frequency of selection for S mutations in the FRs when compared with isotype matched cells in the peripheral blood (p ≤ 0.05), indicating that un-switched B cells in the cervix are under more selective pressure than in the peripheral blood (Fig. 3A).
Figure 3.
Frequency of inferred antigen selection experienced by B cells isolated from the cervix (dark bars) or peripheral blood (light bars). An individual clone was determined to be antigen selected if their p FR (A) or p CDR (B) was below 0.05 when calculated using the multinomial model developed by Lossos et al.46 Significant differences (*) between the frequencies of antigen selection were observed in the cervix when compared with isotype matched B cells in the peripheral blood (p ≤ 0.05).
Identification of clonally related class switched cells in the peripheral blood and cervical mucosa.
Sequence analysis revealed that in addition to site-specific B cells, a number of networks of related sequences sharing identical VH-DH-JH combinations with different CH regions were present among the cervical and peripheral B-cell compartments (Fig. 4). Applying our criteria, a surprisingly high total of 27 of the 156 unique VH sequences from cervical B cells can be traced to a related VH clone from the peripheral blood. This is comparable to the 30 sequences that form CMC-only networks. Considering the observed diversity of the PBMC repertoire, it is possible and even likely that CMC-only networks may also be the progeny of systemic B cells that were not identified in this study. This strongly suggests that the ability of the female genital tract to launch local antibody responses is limited and requires continuous reseeding. This observation is consistent with the limited local antibody responses observed following vaginal immunization with various antigens.50 Alternatively, nearly all of the peripheral blood B-cell networks have crossed into the cervical B-cell population. Only a single PBMC-only network could be formed, consisting of simply two sequences. That the preponderance of related, class switched PBMC clones could also be found in the cervical mucosa indicates that the B-cell homing to the genital tract is a common occurrence. This again may reflect the fact that the cervical samples may have a different cellular composition;51 however, as they were normalized for cDNA this likely reflects true differences in B cells present, ontogeny and somatic evolution.
Figure 4.
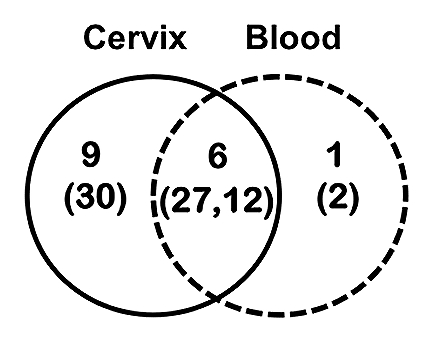
Summary of networks formed between related VH sequences of different isotypes between two immune compartments: the cervical mucosa (solid line) and the peripheral blood (dashed line). The total number of networks identified in each compartment is indicated along with the total number of unique sequences (in parentheses) that collectively form the networks.
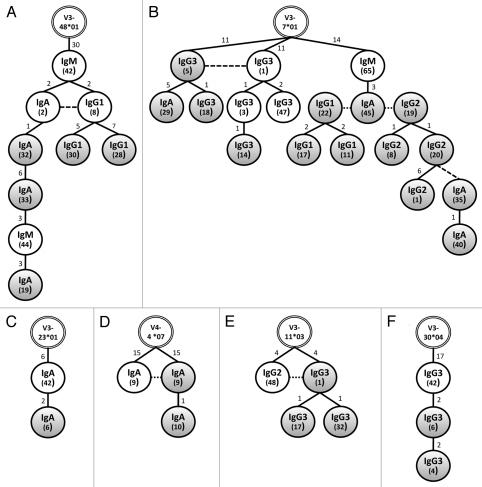
Each of the six compartment spanning networks is depicted in Figure 5. Each isotype appears to be equally represented in these networks with the notable exception of cervical IgM. Indeed, differences in ontogeny appear to hold for the VH genes rearranged as mucosal IgM as none we analyzed were common to both cervix and peripheral blood. While clonal expansion is evident among cervical B cells of the same isotype in five of the six networks (exception of Fig. 5C), class switching appears to occur prior to B-cell migration to the cervix. While there is evidence of class switching among PBMCs in each of the six networks, class switching in cervical B cells is only apparent once in Figure 5B, in that a second class switch event following clonal expansion in the cervix was evident (IgG2-20 to IgA-35).
Figure 5.
Related B-cell networks spanning the cervical mucosa and peripheral blood immune compartments. VH sequences, grouped according to VH loci, were aligned using TCS software with 95% statistical parsimony.47 Networks were formed between sequences with less than eight nucleotide differences. Shown are six networks composed of related, class switched sequences isolated from peripheral blood (white circles) and the cervical mucosa (grey circles). Each network is rooted in the most homologous human germline gene segment (double lined circles at the apex). Numbers in inside the circles within parenthesis indicate the plasmid clone ID. Numbers above each connecting line indicate the number of mutations between individual clones. Dashed lines indicate identical sequences.
Highly mutated VH gene sequences can be found in cervical and systemic spanning B-cell networks. Each VH sequence of the network outlined in Figure 5A is shown in Figure 6. Class switching likely occurred in the systemic compartment before clonal progeny seeded the cervix as identical IgA and IgG1 sequences, sharing common mutations from the closest germline, diversified by accumulating two additional mutations. Additional B cells with further diversification to the IgA and IgG1 isotypes appear in the cervical compartment, each sharing the two mutations found in both parents. Interestingly, all members of the network, with the exception of IgG1 CMC 30, which has two additional mutations, share the 30 mutations from the germline (V3-48*01) observed in the parent PBMC IgM clone. The presence of these shared mutations suggests strongly that affinity maturation occurred in the peripheral blood prior to class switching and diversification.21,52,53 Furthermore, the number of mutations observed in each parent PBMC clone in Figure 5 suggests that the B cells migrating to the cervix have undergone one or more germinal center reactions resulting in somatic mutation and is consistent with our finding that for most isotypes, antigen selection is higher systemically than mucosally. Obviously, the location of IgM PBMC clone 44 as an intermediate between IgA CMC clones 33 and 19 in Figure 5 is in violation of the class switching rules. It is likely that intermediary clones, at least transiently expressed as IgM, persist in circulation following previous class switch events. Hypothetical intermediates were not included in the presented genealogies as the TCS algorithm used generated networks solely based on the sequences present. Alternatively, the large and consistent number of mutations away from germline in multiple classes and sites suggests that polymorphic VH3 genes may be present that are not currently in the databanks. A DNA sequencing effort will be required to elucidate this.
Figure 6.
Confirmation of the relationship between sequences forming network A described in Figure 5. Dashes indicate conserved nucleotides relative to the most homologous germline gene (IGHV3-48*01 and IGHJ6*03). Mutations in bold indicate a mutation that is not shared among all nine related clones.
Discussion
Despite the devastation caused by the 300 million sexually transmitted infections (STIs) estimated to occur annually,5 remarkably few studies have explored the antibody repertoire of the cervical mucosa at a molecular level. Little is known on the ontogeny of antigen specific B-cell responses in the genital mucosa. In particular, how B cells cycle among immune compartments and whether this compartmentalization is supported by common immunoglobulin variable region gene usage in the cervix has not been explored. Evidence reported herein suggests that in addition to antibody extruded to the cervix, humoral immunity in the female genital tract is comprised of a locally resident unique spectrum of B cells, which overlaps with, but is distinct from, the systemic B-cell repertoire. Because our mononuclear cell samples originated from an HEPS individual, our results are likely reflective of an active humoral response occurring in the cervix against numerous STIs including HIV-1.12 Our evidence shows that a considerable portion of B cells in the cervix are descendants of class switched, mutated B cells from circulation. This is likely the result of B-cell homing from the circulation into the mucosa, following systemic germinal center reactions, through yet undefined homing receptors. Consequently, immunity in the human cervix is dependent on the migration of systemic, antigen experienced, isotype switched B cells into the cervix, in addition to the translocation of serum Ig shown previously in reference 54 and 55. The identification of related sequences within the cervical mucosa was not unexpected, as families of related sequences have also been observed within other immune compartments such as the human tonsil,56 intestine,57 spleen58 and subcarinae.59 However, it is not known whether these related sequences reflect local clonal expansion and class switching or are reflective of a limited sample size. The identification of highly related and even identical VH sequences between the cervical mucosa and the peripheral blood compartments is of particular interest because, to the authors' knowledge, this is the first time this has been seen. While highly specific CDR3 clone specific primers have been used to identify related IgG clones in different compartments,60 they do not permit an evaluation of the frequency of the observed clones. Our use of VH family specific primers in the present study, while biased to germline encoded V gene families, is relatively non-biased to combinatorial assemblages; indeed, it is of much lower bias than targeting a somatically combined CDR region as in Thoree et al.60 and this allows us to make conclusions about the regularity of observed clones. The identification of related CMC and PBMC clones does not necessarily imply that the observed mucosal response is of peripheral origin, as B cells migrate to other mucosal tissues via the blood. However, that the overwhelming majority of related class switched clones found in the peripheral blood compartment have related or even identical progeny within the cervical mucosa, and the pattern of hypermutation observed within the networks, suggests strongly that these B cells underwent clonal expansion and class switch in a systemic immune response, and were not simply trafficking from one mucosal site to another.
The VH gene usage is different among the isotypes expressed in the cervix and blood compartments. The use of VH3, and DH gene segments differs significantly between isotype matched IgA and IgG B cells in the cervix relative to the peripheral blood suggesting that much of the cervical B-cell repertoire is comprised of a unique population of B cells. This is consistent with observed differences in both isotype distribution, and antigen specificity of the cervical and peripheral blood compartments.50,54,61,62 In this regard, the observed mucosal compartmentalization adds credence to the presence of HIV-1 specific IgG and IgA in cervicovaginal secretions that is absent from the serum in some HEPS individuals.63,69
The frequency of somatic hypermutation among the VH genes of isotype switched systemic and mucosal B cells is distinct. The B cells of the cervical compartment are oligoclonal with VH genes containing more somatic mutations than those found in the peripheral blood. This is consistent with the VH gene status of B cells from other mucosal sites, which have consistently been shown to have more mutations than peripheral sites, possibly due to the highly antigenic nature of the mucosal environment.20,53 The frequency of hypermutation in cervical IgM B cells was at least as high as that of IgA and IgG B cells in the cervix. This is in contrast to the lower rates of hypermutation reported for IgM B cells in the intestinal mucosa, spleen, salivary gland and the duodenum, relative to isotype controls,21,53,60 Dunn-Walters et al.53 identified related IgM and IgA sequences in the salivary gland in which the IgM clone had accumulated more mutations than the class switched clone. IgM plasma cells in the intestine have also been shown to have similar frequencies of mutation as IgA plasma cells.64 Therefore, IgM B cells that persist following germinal center reactions clearly accumulate mutations even after class switching. In the intestine, switching from sIgM to sIgA occurs in immune inductive sites such as mucosal Peyer's patches;65 however, because the genital tract lacks equivalent immune inductive sites, isotype switching from IgM B cells in the cervix may require homing to a local lymph node thus occurring at a slower rate. The resulting persistence of IgM-expressing B cells in the local environment would allow somatic mutations to accumulate and could also account for our observation that only IgM B cells appear to be more antigen-experienced in the cervix compared to the blood.
Furthermore, no significant differences were observed between VH, DH and JH gene segment use in IgM B cells in the cervix when compared with those in peripheral blood, suggesting that either both compartments are composed of overlapping repertoires, or the selective pressures that shape VH gene expression in isotype switched cervical B cells are less pronounced in non-switched B cells. The fact that no related IgM sequences were shared between the cervix and peripheral blood compartments, despite an increased sample size, implies that the cervical IgM compartment is separate from the peripheral blood, and fundamental differences in the mechanisms of B-cell ontogeny in the cervix are responsible for the observed differences in somatic mutation and affinity maturation.
Alternatively, class switch recombination and affinity maturation may either have different requirements at the mucosal surface of the cervix, or occur outside germinal centers as has been shown in animal models.66
These data support a two-tiered antibody protection strategy in the genital tract. Similar to the strategy proposed for the intestinal mucosa67 an oligodiverse, highly mutated antibody repertoire exists within the cervical mucosa that is consistently repopulated by affinity matured class-switched B cells from the periphery. However, despite some similarities, differences in isotype ratios between the intestinal and genital tract implies there are considerable differences in both B-cell populations.5,13,49,68 In the gut where IgA is dominant, this “innate-like” response is mediated by a restricted IgA repertoire exhibiting low affinity, yet broad reactivity.67 In the cervix, where IgG is dominant, we found that the IgA response appears polyclonal with an inferred frequency of affinity maturation comparable to, and in some cases higher than, other isotypes. Furthermore, the apparent clonal expansion of cervical IgA bearing B cells in four of the compartment spanning networks suggests that the role played by IgA in the genital tract may be more adaptive in nature than the innate-like role it plays in other mucosal tissues. This is consistent with the extensive somatic mutation recently observed by others in the CDRs of gp41 specific Fabs selected from phage libraries derived from mucosal B cells of HEPS individuals.12 These IgA-derived antibodies are neutralizing in transcytosis and CD4 based assays and one of the four Fabs utilizes a VH3 allele with and an extended CDR3 characteristic of envelope-specific HIV neutralizing antibodies. Similarly, many of the IgA VH3 alleles we identified from the cervix of our HEPS patient appear to have undergone extensive affinity maturation in an antigen-driven process. The repeated exposure to HIV-1 in these individuals may result in a local enrichment of antigen experienced IgA bearing B cells and could play a role in the prevention of HIV-1. Additionally, the observed oligoclonality and low frequency of antigen selection and hypermutation of IgG1 B cells presented here, coupled with the subclass dominance of IgG1 in cervicovaginal secretions,54 suggests that in the cervix, IgG1 may mirror to the “innate-like” role played by IgA in the gut.
In conclusion, we characterized the VH genes expressed in the B-cell repertoire of the human cervical mucosa. We present molecular evidence for the compartmentalization of cervical B-cell populations from the peripheral blood repertoire in a HEPS individual. Moreover, we show that many class switched B cells from circulation appear to relocate to the genital tract and analysis of their V genes allows us to form compartment and isotype spanning networks. These molecular data, along with the protection afforded by systemically administered HPV vaccines,69,70 and findings by others,5 collectively suggest that vaccines capable of inducing a systemic antibody response should be an effective method of inducing locally protective antibody. Indeed, systemic immunization has been shown to induce high levels of specific antibodies in the genitourinary tract.5,14,17,71 Lastly, homing of B cells to the cervix may allow rescue of B cells with potentially auto-reactive specificity to forbidden epitopes on HIV-1 generated by systemic immunization to the external mucosal environment.72 The authors note that some level of caution should be taken in extrapolating somatic mutation and mucosal-systemic B-cell trafficking patterns from repertoire analysis conducted on B cells from a single individual. We recognize that a larger and more robust study involving samples from multiple subjects collected at different time points can now be done using cutting-edge technology; however, this study remains as a comparator for the current technology. These data show a complex pattern of B-cell sharing and compartmentalization and further repertoire characterization by ultra-deep pyrosequencing39 and assembly of monoclonal antibodies from the V-genes73,74 of locally present plasmablasts from this cohort is anticipated and will further shed light on local immune responses in the cervix.
Materials and Methods
Subject.
Peripheral blood mononuclear cell (PBMC) and cervical mononuclear cell (CMC) samples were collected in parallel from a HEPS commercial sex worker from Nairobi, Kenya. This individual is a member of a well-characterized cohort of commercial sex workers in the Pumwani area of Nairobi41 and has annually tested seronegative for HIV-1, and confirmed by PCR, since testing began in 1985. Despite counselling, it is estimated these women have approximately 64 encounters with HIV-1 every year through unprotected sex.41
Sample collection.
PBMC and CMC samples were harvested as part of a routine survey in February 2007 under ethical approval by the University of Nairobi. Both PBMC and CMC samples were collected using previously established methods.42 Briefly, PBMCs were collected by Ficoll-Hypaque gradient centrifugation and CMCs were harvested using a clinical cytobrush (Histobrush; Spectum Labs). To prevent contamination with peripheral blood, CMC samples containing any visual blood were discarded. Cells were washed and re-suspended into RNA lysis buffer (QIAGEN®) and stored in liquid nitrogen.
PCR.
Total RNA was recovered from the CMC and PBMC samples using the RNeasy Mini Kit (QIAGEN®) according to manufacturer's instructions. cDNA was synthesized from equivalent quantities of RNA using the Thermoscript RT-PCR system (Invitrogen) with an oligo dT primer according to manufacturer's instructions. cDNA was amplified with the Expand High Fidelity PCR System (Roche) in 50 µl using 600 nM of sense olignucleotide primer (VhF) specific for FR-1 of VH3, and 600 nM of one of 5 anti-sense primers specific for the 5′ end of Cµ, Cγ1, Cγ2a, Cγ3 or Cα43 (Table 1). After initial hot start, amplification consisted of ten cycles of 20 seconds at 94°C, 30 seconds at 55°C and 45 seconds at 72°C, followed by 30 cycles of 30 seconds at 94°C, 30 seconds at 55°C and 45 seconds at 72°C (5 second increase in incubation time with each cycle), followed by a final extension for 7 min at 72°C.
Table 1.
Sequences of oligonucleotide primers used in the PCR amplification of VH sequences
| Primera | Sequence 5′ to 3′ |
| VhF | CAG GTG CAG CTG CTC GAG TCT GG |
| IgG1 Reverse | CAT GTA CTA GTT TTG TCA CAA GAT TTG GG |
| IgG2a Reverse | TCT ACA CTA GTT TTG CGC TCA ACT GTC TT |
| IgG3 Reverse | TGT GTG ACT AGT GTC ACC AAG TGG GGT TTT |
| IgA Reverse | CTA GTG ACC TTG GGG CTG GTC GGG GAT GC |
| IgM Reverse | CTC ACA CTA GTA GGC AGC TCA GCA ATC AC |
Sequences were adapted from Barbas, et al.43 (2001).
Cloning and sequencing.
The VH PCR products were gel purified (QIAGEN® Gel Extraction Kit) and cloned into pCR®2.1-TOPO-TA™ (Invitrogen) as per manufacturer's instructions. Individual bacterial colonies were chosen on LB-AMP (100 µg/ml), expanded in overnight 2 ml bacterial cultures with ampicillin (50 µg/ml), and the individual plasmids isolated using QIAprep® Spin Miniprep kits (QIAGEN®). The VH inserts were sequenced on both strands with T7 (5′-TAA TAC GAC TCA CTA TAG GG-3′) and M13R (5′-CAG GAA ACA GCT ATG AC-3′) primers (Invitrogen) with an ABI Prism® 3730XL DNA sequencer using Applied Biosystems BigDye™ Terminator v3.1.
Identification of Ig germline sequences.
Each of the VH consensus nucleotide sequences were trimmed to remove all residues upstream of FR-1 and downstream of FR-4, then compared with known human germ line VH genes to identify the V, J and D segments using IMGT/V-Quest.44 CDRs and FRs were identified according to numbering of the inferred amino acid sequences according to Kabat et al.45
Molecular analysis of VH genes.
The multinomial model developed by Lossos et al.46 was used to estimate the antigen selection experienced by each clone. The number of observed replacement (R) and silent (S) mutations in FRs 1–3 and CDRs 1 and 2 from each clone were input along with the nucleotide sequence of the most homologous germline gene to estimate the probability (p) that the observed excess or scarcity of R mutations observed in the CDRs or FRs respectively were generated solely through random mutation.46 A clone was considered to have experienced antigen selection in the FRs or CDRs respectively if either p FR or p CDR < 0.05. Mean p FR and p CDR values were also determined for each isotype within both immune compartments. While this model does not take into account biases caused by the intrinsic mutability of human CDRs, it does provide a good estimation of the selection pressure experienced by antibody genes.
All sequences were grouped according to VH locus and networks of related clones were identified using TCS software developed by Clement et al.47 with 95% statistical parsimony. Networks were formed between sequences with less than eight nucleotide mutations.
Statistical analysis.
Continuous data was analyzed using a Mann-Whitney U test with GraphPad Prism software as the data did not follow the Gaussian distribution. χ2 tests were used to compare proportions of V, D and J gene segment usage between the cervix and peripheral blood within isotypes. Diversity of CMC and PBMC samples were analyzed by comparing frequencies of unique V-D-J rearrangments found within isotypes using Fisher's exact test. Observed differences were considered significant when p ≤ 0.05.
Acknowledgements
F. Plummer is a Tier I Canada Research Chair. This work was supported by funding from the Public Health Agency of Canada (J.D.B., F.P.), the Bill and Melinda Gates Foundation, the Canadian Institute of Health Research, and from resource cores supported by the CBRN Research and Technology Initiative (CRTI). R.G.G. was supported by a University of Manitoba Graduate Student Fellowship.
Abbreviations
- CDR
complementarity determining region
- CH
heavy chain constant region
- CMC
cervical mononuclear cell
- DH
heavy chain diversity region
- FR
framework region
- HEPS
highly exposed yet persistently seronegative
- HIV-1
human immunodeficiency virus type-1
- Ig
immunoglobulin
- JH
heavy chain junction region
- VH
heavy chain variable region
- PBMC
peripheral blood mononuclear cell
- R
replacement
- S
silent
- STI
sexually transmitted infection
References
- 1.Virgin HW, Walker BD. Immunology and the elusive AIDS vaccine. Nature. 2010;464:224–231. doi: 10.1038/nature08898. [DOI] [PubMed] [Google Scholar]
- 2.Richman DD, Wrin T, Little SJ, Petropoulos CJ. Rapid evolution of the neutralizing antibody response to HIV type 1 infection. Proc Natl Acad Sci USA. 2003;100:4144–4149. doi: 10.1073/pnas.0630530100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wei X, Decker JM, Wang S, Hui H, Kappes JC, Wu X, et al. Antibody neutralization and escape by HIV-1. Nature. 2003;422:307–312. doi: 10.1038/nature01470. [DOI] [PubMed] [Google Scholar]
- 4.Mascola JR, Stiegler G, VanCott TC, Katinger H, Carpenter CB, Hanson CE, et al. Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nat Med. 2000;6:207–210. doi: 10.1038/72318. [DOI] [PubMed] [Google Scholar]
- 5.Moldoveanu Z, Huang WQ, Kulhavy R, Plate MS, Mestecky J. Human male genital tract secretions: both mucosal and systemic immune compartments contribute to the humoral immunity. J Immunol. 2005;175:4127–4136. doi: 10.4049/jimmunol.175.6.4127. [DOI] [PubMed] [Google Scholar]
- 6.Kaul R, Trabattoni D, Bwayo JJ, Arienti D, Zagliani A, Mwangi FM, et al. HIV-1 specific mucosal IgA in a cohort of HIV-1 resistant Kenyan sex workers. AIDS. 1999;13:23–29. doi: 10.1097/00002030-199901140-00004. [DOI] [PubMed] [Google Scholar]
- 7.Bélèc L, Ghys PD, Hocini H, Nkengasong JN, Tranchot-Diallo J, Diallo MO, et al. Cervicovaginal secretory antibodies to human immunodeficiency virus type 1 (HIV-1) that block viral transcytosis through tight epithelial barriers in highly exposed HIV-1 seronegative African women. J Infect Dis. 2001;184:1412–1422. doi: 10.1086/324375. [DOI] [PubMed] [Google Scholar]
- 8.Broliden K, Hinkula J, Devito C, Kiama P, Kimani J, Trabbatoni D, et al. Functional HIV-1 specific IgA antibodies in HIV-1 exposed, persistently IgG seronegative female sex workers. Immunol Lett. 2001;79:29–36. doi: 10.1016/s0165-2478(01)00263-2. [DOI] [PubMed] [Google Scholar]
- 9.Buchacz K, Parekh BS, Padian NS, van der Straten A, Phillips S, Jonte J, et al. HIV-specific IgG in cervicovaginal secretions of exposed HIV-uninfected female sexual partners of HIV-infected men. AIDS Res Hum Retroviruses. 2001;17:1689–1693. doi: 10.1089/08892220152741388. [DOI] [PubMed] [Google Scholar]
- 10.Nguyen M, Pean P, Lopalco L, Nouhin J, Phoung V, Ly N, et al. HIV-specific antibodies but not T-cell responses are associated with protection in seronegative partners of HIV-1-infected individuals in Cambodia. J Acq Immun Def Synd. 2006;42:412–419. doi: 10.1097/01.qai.0000222289.97825.35. [DOI] [PubMed] [Google Scholar]
- 11.Alexander R, Mestecky J. Neutralizing antibodies in mucosal secretions: IgG or IgA? Curr HIV Res. 2007;5:588–593. doi: 10.2174/157016207782418452. [DOI] [PubMed] [Google Scholar]
- 12.Tudor D, Derrien M, Diomede L, Drillet AS, Houimel M, Moog C, et al. HIV-1-gp41 specific monoclonal mucosal IgAs derived from highly exposed but IgG-seronegative individuals block HIV-1 epithelial transcytosis and neutralize CD4+ cell infection: an IgA gene and functional analysis. Muc Immunol. 2009;4:412–426. doi: 10.1038/mi.2009.89. [DOI] [PubMed] [Google Scholar]
- 13.Mestecky J, Moldoveanu Z, Russell MW. Immunologic uniqueness of the genital tract: challenge for vaccine development. Am J Reprod Immunol. 2005;53:208–214. doi: 10.1111/j.1600-0897.2005.00267.x. [DOI] [PubMed] [Google Scholar]
- 14.Bouvet JP, Bélèc L, Pirès R, Pillot J. Immunoglobulin G antibodies in human vaginal secretions after parenteral vaccination. Infect Immun. 1994;62:3957–3961. doi: 10.1128/iai.62.9.3957-3961.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bergquist C, Johansson EL, Lagergard T, Holmgren J, Rudin A. Intranasal vaccination of humans with recombinant cholera toxin B subunit induces systemic and local antibody responses in the upper respiratory tract and the vagina. Infect Immun. 1997;65:2676–2684. doi: 10.1128/iai.65.7.2676-2684.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crowley-Nowick PA, Bell MC, Brockwell R, Edwards RP, Chen S, Patridge E, et al. Rectal immunization for induction of specific antibody in the genital tract of women. J Clin Immunol. 1997;17:370–379. doi: 10.1023/a:1027312223474. [DOI] [PubMed] [Google Scholar]
- 17.Ashley RI, Crisostomo FM, Doss M, Sekulovich RE, Burke RI, Shaughnessy M, et al. Cervical antibody responses to a herpes simplex virus type 2 glycoprotein subunit vaccine. J Infect Dis. 1998;178:1–7. doi: 10.1086/515611. [DOI] [PubMed] [Google Scholar]
- 18.Kutteh WH, Kantele A, Moldoveanu Z, Crowley-Nowick PA, Mestecky J. Induction of specific immune responses in the genital tract of women after oral or rectal immunization and rectal boosting with Salmonella typhi Ty21a vaccine. J Reprod Immunol. 2001;52:61–75. doi: 10.1016/s0165-0378(01)00109-7. [DOI] [PubMed] [Google Scholar]
- 19.Dunn-Walters DK, Boursier L, Spencer J. Hypermutation, diversity and dissemination of human intestinal lamina propria plasma cells. Eur J Immunol. 1997;27:2959–2964. doi: 10.1002/eji.1830271131. [DOI] [PubMed] [Google Scholar]
- 20.Dunn-Walters DK, Isaacson PG, Spencer J. Sequence analysis of human Ig VH genes indicates that ileal laminal propria plasma cells are derived from Peyer's patches. Eur J Immunol. 1997;27:463–467. doi: 10.1002/eji.1830270217. [DOI] [PubMed] [Google Scholar]
- 21.Boursier L, Dunn-Walters DK, Spencer J. Characteristics of IgVH genes used by human intestinal plasma cells from childhood. Immunology. 1999;97:558–564. doi: 10.1046/j.1365-2567.1999.00843.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Croce CM, Shander M, Martinis J, Cicurel L, D'Ancona GG, Dolby TW, et al. Chromosomal location of the genes for human immunoglobulin heavy chains. Proc Natl Acad Sci. 1979;76:3416–3419. doi: 10.1073/pnas.76.7.3416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matsuda F, Shin EK, Nagaoka H, Matsumura R, Haino M, Fukita Y, et al. Structure and physical map of 64 variable segments in the 3′ 0.8-megabase region of the human immunoglobulin heavy-chain locus. Nat Genet. 1993;3:88–94. doi: 10.1038/ng0193-88. [DOI] [PubMed] [Google Scholar]
- 24.Cook GP, Tomlinson IM. The human immunoglobulin VH repertoire. Immunol Today. 1995;16:237–242. doi: 10.1016/0167-5699(95)80166-9. [DOI] [PubMed] [Google Scholar]
- 25.Matsuda F, Ishii K, Bourvagnet P, Kuma K, Hayashida H, Miyata T, Honjo T. The complete nucleotide sequence of the human immunoglobulin heavy chain variable region locus. J Exp Med. 1998;188:2151–2162. doi: 10.1084/jem.188.11.2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Siebenlist U, Ravetch JV, Korsmeyer S, Waldmann T, Leder P. Human immunoglobulin D segments encoded in tandem multigenic families. Nature. 1981;294:631–635. doi: 10.1038/294631a0. [DOI] [PubMed] [Google Scholar]
- 27.Corbett SJ, Tomlinson IM, Sonnhammer E, Buck D, Winter G. Sequence of the human immunoglobulin diversity (D) segment locus: a systematic analysis provides no evidence for the use of DIR segments, inverted D segments, ‘minor' D segments or D-D recombinations. J Mol Biol. 1997;270:587–597. doi: 10.1006/jmbi.1997.1141. [DOI] [PubMed] [Google Scholar]
- 28.Ravetch JV, Siebenlist U, Korsmeyer S, Waldmann T, Leder P. Structure of the human immunoglobulin mu locus: characterization of embryonic and rearranged J and D genes. Cell. 1981;27:583–591. doi: 10.1016/0092-8674(81)90400-1. [DOI] [PubMed] [Google Scholar]
- 29.Lefranc MP, Lefranc G, Rabbitts TH. Inherited deletion of immunoglobulin heavy chain constant region genes in normal human individuals. Nature. 1982;300:760–762. doi: 10.1038/300760a0. [DOI] [PubMed] [Google Scholar]
- 30.Flanagan JG, Rabbitts TH. Arrangement of human immunoglobulin heavy chain constant region genes implies evolutionary duplication of a gene segment containing gamma, epsilon and alpha genes. Nature. 1982;300:709–713. doi: 10.1038/300709a0. [DOI] [PubMed] [Google Scholar]
- 31.Tonegawa S. Somatic generation of antibody diversity. Nature. 1983;302:575–581. doi: 10.1038/302575a0. [DOI] [PubMed] [Google Scholar]
- 32.French DL, Laskov R, Scharff MD. The role of somatic hypermutation in the generation of antibody diversity. Science. 1989;244:1152–1157. doi: 10.1126/science.2658060. [DOI] [PubMed] [Google Scholar]
- 33.Lederberg J. Genes and antibodies. Science. 1959;129:1649–1653. doi: 10.1126/science.129.3364.1649. [DOI] [PubMed] [Google Scholar]
- 34.Berek C. The development of B cells and the B-cell repertoire in the microenvironment of the germinal center. Immunol Rev. 1992;126:5–19. doi: 10.1111/j.1600-065x.1992.tb00628.x. [DOI] [PubMed] [Google Scholar]
- 35.Fish S, Zenowich E, Fleming M, Manser T. Molecular analysis of original antigenic sin. I. Clonal selection, somatic mutation and isotype switching during a memory B cell response. J Exp Med. 1989;170:1191–1209. doi: 10.1084/jem.170.4.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kraj P, Rao SP, Glas AM, Hardy RR, Milner EC, Silberstein LE. The human heavy chain Ig V region gene repertoire is biased at all stages of B cell ontogeny. J Immunol. 1997;158:5824–5832. [PubMed] [Google Scholar]
- 37.Seifert M, Küppers R. Molecular footprints of a germinal center derivation of human IgM+(IgD+) CD27+ B cells and the dynamics of memory B cell generation. J Exp Med. 2009;206:2659–2669. doi: 10.1084/jem.20091087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Campbell PJ, Stephens PJ, Pleasance ED, O'Meara S, Li H, Santarius T, et al. Identification of a somatically acquired rearrangements in cancer using genome-wide massively parallel paired-end sequencing. Nat Genet. 2008;40:722–729. doi: 10.1038/ng.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Obermeier B, Mentele R, Malotka J, Josef K, Kümpfel T, Wekerle H, et al. Matching of oligoclonal immunoglobulin transcriptomes and proteomes of cerebrospinal fluid in multiple sclerosis. Nat Med. 2008;14:688–693. doi: 10.1038/nm1714. [DOI] [PubMed] [Google Scholar]
- 40.Fraser NLW, Rowley G, Field M, Stott DI. The VH gene repertoire of splenic B cells and somatic hypermutation in systemic lupus erythematosus. Arthritis Res Ther. 2002;5:114–121. doi: 10.1186/ar627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fowke KR, Nagelkerke NJD, Kimani J, Simonsen JN, Anzala AO, Bwayo JJ, et al. Resistance to HIV-1 infection among persistently seronegative prostitutes in Nairobi, Kenya. Lancet. 1996;348:1347–1351. doi: 10.1016/S0140-6736(95)12269-2. [DOI] [PubMed] [Google Scholar]
- 42.Kaul R, Plummer FA, Kimani J, Dong T, Kiama P, Rostron T, et al. HIV-1 specific mucosal CD8+ lymphocyte responses in the cervix of HIV-1 resistant prostitutes in Nairobi. J Immunol. 2000;164:1602–1611. doi: 10.4049/jimmunol.164.3.1602. [DOI] [PubMed] [Google Scholar]
- 43.Barbas CF, III, Burton DR, Scott JK, Silverman GJ. Phage Display: A Laboratory Manual. In: Barbaras CF, editor. Cold Spring Harbor. NY: Cold Spring Harbor Laboratory Press; 2001. p. 736. [Google Scholar]
- 44.Brochet X, Lefranc MP, Giudicelli V. IMGT/V-QUEST: the highly customized and integrated system for Ig and TR standardized V-J and V-D-J sequence analysis. Nucleic Acids Res. 2008;36:503–508. doi: 10.1093/nar/gkn316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kabat EA, Wu TT, Perry HM, Gottesman KS, Foeller C. Sequences of proteins of immunological interest. Fifth edition. Washington DC: US Department of Health and Human Services; 1991. [Google Scholar]
- 46.Lossos IS, Tibshirani R, Narasimhan B, Levy R. The inference of antigen selection on Ig genes. J Immunol. 2000;165:5122–5126. doi: 10.4049/jimmunol.165.9.5122. [DOI] [PubMed] [Google Scholar]
- 47.Clement M, Posada D, Crandall KA. TCS: a computer program to estimate gene genealogies. Mol Ecol. 2000;9:1657–1660. doi: 10.1046/j.1365-294x.2000.01020.x. [DOI] [PubMed] [Google Scholar]
- 48.Hougs L, Juul L, Ditzel HJ, Heilmann C, Svejgaard A, Barington T. The first dose of a Haemophilus influenzae type b conjugate vaccine reactivates memory B cells: Evidence for extensive clonal selection, intraclonal affinity maturation and multiple isotype switches to IgA2. J Immunol. 1999;162:224–237. [PubMed] [Google Scholar]
- 49.Kutteh WH, Hatch KD, Blackwell RE, Mestecky J. Secretory immune system of the female reproductive tract: I. Immunoglobulin and secretory component-containing cells. Obstet Gynecol. 1988;71:56–60. [PubMed] [Google Scholar]
- 50.Russell MW, Mestecky J. Humoral immune responses to microbial infections in the genital tract. Microbes Infect. 2002;4:667–677. doi: 10.1016/s1286-4579(02)01585-x. [DOI] [PubMed] [Google Scholar]
- 51.Horton RE, Kaefer N, Songok E, Guijon FB, Kettaf N, Boucher G, et al. A comparative analysis of gene expression patterns and cell phenotypes between cervical and peripheral blood mononuclear cells. PLoS ONE. 2009;4:8293. doi: 10.1371/journal.pone.0008293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stoel M, Jiang HQ, van Diemen CC, Bun JCAM, Dammers P, Thurnheer MC, et al. Restricted IgA repertoire in both B-1 and B-2 cell-derived gut plasmablasts. J Immunol. 2005;174:1046–1054. doi: 10.4049/jimmunol.174.2.1046. [DOI] [PubMed] [Google Scholar]
- 53.Dunn-Walters DK, Hackett M, Boursier L, Ciclitira PJ, Morgan P, Challacombe SJ, et al. Characteristics of human IgA and IgM genes used by plasma cells in the salivary gland resemble those used in the duodenum but not those used in the spleen. J Immunol. 2000;164:1595–1601. doi: 10.4049/jimmunol.164.3.1595. [DOI] [PubMed] [Google Scholar]
- 54.Hocini H, Barra A, Bélec L, Iscaki S, Preud'Homme JL, Pillot J, et al. Systemic and secretory humoral immunity in the normal human vaginal tract. Scand J Immunol. 1995;42:269–274. doi: 10.1111/j.1365-3083.1995.tb03653.x. [DOI] [PubMed] [Google Scholar]
- 55.Bouvet JP, Fischetti VA. Diversity of antibody-mediated immunity at the mucosal barrier. Infect Immun. 1999;67:2687–2691. doi: 10.1128/iai.67.6.2687-2691.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chapman CJ, Mockridge CI, Hamblin TJ, Stevenson FK. Tracking of the V4–34 (VH4–21) gene in human tonsil reveals clonal isotype switch events and a highly variable degree of somatic hypermutation. Clin Exp Immunol. 1996;105:360–368. doi: 10.1046/j.1365-2249.1996.d01-769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Boursier L, Farstad IN, Mellembakken JR, Brandtzaeg P, Spencer J. IgVH gene analysis suggests that peritoneal B cells do not contribute to the gut immune system in man. Eur J Immunol. 2002;32:2427–2436. doi: 10.1002/1521-4141(200209)32:9<2427::AID-IMMU2427>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 58.Varade WS, Insel RA. Isolation of germinal center like events from human spleen RNA: somatic hypermutation of a clonally related VH6DJH rearrangement expressed with IgM, IgG and IgA. J Clin Invest. 1993;91:1838–1842. doi: 10.1172/JCI116397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Snow RE, Djukanovic R, Stevenson FK. Analysis of immunoglobulin E VH transcripts in a bronchial biopsy of an asthmatic patient confirms bias towards VH5 and indicates local clonal expansion, somatic mutation and isotype switch events. Immunology. 1999;98:646–651. doi: 10.1046/j.1365-2567.1999.00910.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Thoree VC, Golby SJC, Boursier L, Hackett M, Dunn-Walters DK, Sanderson JD, et al. Related IgA1 and IgG producing cells in blood and diseased mucosa in ulcerative colitis. Gut. 2002;51:44–50. doi: 10.1136/gut.51.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ashley RI, Corey L, Dalessio J, Wilson P, Remington M, Barnum G, Trethewey P. Protein-specific cervical antibody responses to primary genital herpes simplex virus type 2 infections. J Infect Dis. 1994;170:20–26. doi: 10.1093/infdis/170.1.20. [DOI] [PubMed] [Google Scholar]
- 62.Bélèc L, Dupré T, Prazuck T, Tévi-Bénissan C, Kanga JM, Pathey O, et al. Cervicovaginal overproduction of specific IgG to human immunodeficiency virus (HIV) contrasts with normal or impaired IgA local response in HIV infection. J Infect Dis. 1995;172:691–697. doi: 10.1093/infdis/172.3.691. [DOI] [PubMed] [Google Scholar]
- 63.Bélèc L, Tévi-Bénissan C, Lu XS, Prazuck T, Pillot J. Local synthesis of IgG antibodies to HIV within the female and male genital tracts during asymptomatic and pre-AIDS stages of HIV infection. AIDS Res Hum Retroviruses. 1995;11:719–729. doi: 10.1089/aid.1995.11.719. [DOI] [PubMed] [Google Scholar]
- 64.Fischer M, Küppers R. Human IgA- and IgM-secreting intestinal plasma cells carry heavily mutated VH regions genes. Eur J Immunol. 1998;28:2971–2977. doi: 10.1002/(SICI)1521-4141(199809)28:09<2971::AID-IMMU2971>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 65.Mestecky J. Humoral immune responses to the human immunodeficiency virus type-1 (HIV-1) in the genital tract compared to other mucosal sites. J Reprod Immunol. 2006;75:1–17. doi: 10.1016/j.jri.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 66.Toellner KM, Jenkinson WE, Taylor DR, Khan M, Sze DMY, Sansom DM, et al. Low-level hypermutation in T cell-independent germinal centers compared with high mutation rates associated with T cell-dependent germinal centers. J Exp Med. 2002;195:383–389. doi: 10.1084/jem.20011112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Brandtzaeg P. Induction of secretory immunity and memory at mucosal surfaces. Vaccine. 2007;25:5467–5484. doi: 10.1016/j.vaccine.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 68.Crowley-Nowick PA, Bell M, Edwards RP, McCallister D, Gore H, Kanbour-Shakir A, et al. Normal uterine cervix: characterization of isolated lymphocyte phenotypes and immunoglobulin secretion. Am J Reprod Immunol. 1995;34:241–247. doi: 10.1111/j.1600-0897.1995.tb00948.x. [DOI] [PubMed] [Google Scholar]
- 69.Torre GL, de Waure C, Chiaradia G, Mannocci A, Ricciardi W. HPV vaccine efficacy in preventing persistent cervical HPV infection: a systemic review and meta-analysis. Vaccine. 2007;25:8352–8358. doi: 10.1016/j.vaccine.2007.09.027. [DOI] [PubMed] [Google Scholar]
- 70.Paavonen J, Naud P, Salmeron J, Wheeler CM, Chow SN, Apter D, et al. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double blind, randomized study in young women. Lancet. 2009;374:301–314. doi: 10.1016/S0140-6736(09)61248-4. [DOI] [PubMed] [Google Scholar]
- 71.Wu HY, Abdu S, Stinson D, Russell MW. Generation of female genital tract antibody responses by local or central (common) mucosal immunization. Infect Immun. 2000;68:5539–5545. doi: 10.1128/iai.68.10.5539-5545.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Xiao X, Chen W, Feng Y, Zhu Z, Prabakaran P, Wang Y, et al. Germline-like predecessors of broadly neutralizing antibodies lack measurable binding to HIV-1 envelope glycoproteins: implications for evasion of immune responses and design of vaccine immunogens. Biochem Biophys Res Commun. 2009;390:404–409. doi: 10.1016/j.bbrc.2009.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Di Niro R, Mesin L, Raki M, Nai-Ying Z, Lund-Johansen F, Lundin KEA, et al. Rapid generation of rotavirus-specific human monoclonal antibodies from small-intestinal mucosa. J Immunol. 2010;185:5377–5383. doi: 10.4049/jimmunol.1001587. [DOI] [PubMed] [Google Scholar]
- 74.Reddy ST, Ge X, Miklos AE, Hughes RA, Kang SH, Hoi KH, et al. Monoclonal antibodies isolated without screening by analyzing the variable-gene repertoire of plasma cells. Nat Biotechnol. 2010;28:965–969. doi: 10.1038/nbt.1673. [DOI] [PubMed] [Google Scholar]