DNA banking has the potential to provide critical information to relatives of patients with cancer. Despite this, it is not being used by palliative care oncologists.
Abstract
Purpose:
The availability of genetic tests for cancer susceptibility is increasing. Current tests, however, have limited clinical sensitivity. Even when clinically valid tests are available, the genetic counseling and informed consent process might not be feasible for dying patients with cancer. DNA banking preserves the opportunity for future research or clinical testing and may provide critical opportunities for surviving relatives. This study explored the current practices and potential for DNA banking for cancer susceptibility among oncologists specializing in palliative care.
Methods:
Palliative care oncologists actively providing clinical care for dying patients with cancer were recruited for an online survey. Descriptive statistics for DNA banking practices, perceived qualification to recommend banking, and potential predictors were assessed.
Results:
Data were collected from 49 physicians (37% recruitment rate). Eighty percent reported assessing at least some patients for genetic cancer susceptibility in the past 12 months. No participants reported banking DNA for patients in the past 12 months. Only 5% reported feeling at least somewhat qualified to order DNA banking. A Web-based risk assessment tool and genetic counselor on staff were perceived as the most helpful potential resources.
Conclusion:
Despite its potential, DNA banking is not being used by palliative care oncologists.
Introduction
Identifying genetic susceptibility for cancer is an accepted part of current oncology practice.1 For some genetic susceptibilities (eg, familial medullary thyroid cancer, familial adenomatous polyposis, and hereditary breast and ovarian cancer), the clinical validity and utility of current genetic tests support routine genetic testing in the context of informed consent and genetic counseling. For many patients, however, current clinical genetic tests lack validity and/or utility.2 For example, fewer than half of women with hereditary breast cancer have mutations in the BRCA1 or BRCA2 genes.3
However, the rapid increase in gene discovery and testing technology suggests future testing could be helpful for surviving family members. This may be particularly relevant for family members of the 5% to 10% of patients with autosomal dominant cancer risk.4 Approximately half of these patients' children and siblings will have strong cancer risk, and half will not. For these families, DNA banking preserves the opportunity to identify a genetic marker after patients have died. In contrast to testing for a known cancer susceptibility gene, DNA banking involves storing a tissue sample containing constitutional DNA (usually peripheral blood) so that testing can proceed in the future, after the patient has died.
There may be fiscal reasons to consider DNA banking at the end of life as well. Because the primary medical reason for genetics assessment at the end of life is for the patient's family members, it may not be a covered service by insurers as it is not deemed medically necessary. DNA banking may be an attractive option in light of this fiscal concern because it is usually much cheaper (about $100) compared with genetic testing, which often costs several thousand dollars.
Although genetics assessment and DNA banking have been proposed as valuable end-of-life services,5–7 to date very little is known about actual clinical practice. We recently reported results from interviews of dying patients with cancer that suggest limited, if any, use of DNA banking, despite approximately one in five of these patients potentially having hereditary cancer risk.8 Findings from this study came from a single institution and had a limited sample size, precluding generalizable conclusions about physician practices. However, since the study occurred in a large academic institution with on-site clinical genetics services, the lack of DNA banking could point to a widespread gap in services.
Previous studies have focused on physician knowledge, attitudes, and behaviors with respect to cancer susceptibility testing.9,10 These studies show high perceived qualifications among oncologists to order genetic tests, and more knowledge about cancer susceptibility tests relative to other specialists. To our knowledge, however, no studies have assessed practices and perceived qualifications with respect to DNA banking. The primary objective of this report is to describe current physician practices regarding DNA banking among dying patients with cancer. We hypothesized that a low proportion (∼5%) of physicians would report recommending DNA banking for their patients. As we have found no previous studies that address this research question, we focused on providers specializing in both oncology and palliative care; this subset of physicians may be in the best position to identify the need for DNA banking among dying cancer patients. We also describe physicians' self-efficacy to facilitate DNA banking and what resources or tools they feel would be helpful.
Methods
In spring, 2008, we conducted a national cross-sectional descriptive study of palliative care oncologists' practices regarding DNA banking for cancer susceptibility. The American Board of Hospice and Palliative Medicine (ABHPM) is the accrediting board for palliative care and maintains a listing of currently board-certified physicians. With institutional review board approval from ABHPM, we obtained a list of contact information for physicians currently certified in both oncology and palliative medicine (N = 133). Study inclusion criteria were (1) being board-certified in both palliative care and oncology, and (2) having cared for end-of-life patients with cancer during the previous 12 months. Physicians were sent an e-mail invitation to participate in a brief survey. The survey was administered online using SurveyMonkey, and responses were imported into a Microsoft Office Excel 2003 database without personal identifiers.
If physicians did not complete the survey online within 2 weeks, a second e-mail was sent. If there was still no response, physicians were faxed the invitation and survey. Finally, if no response was obtained, a paper copy was mailed up to two times. All forms of communication originated from an investigator who is board-certified in both oncology and palliative medicine and is well-known and respected among this peer group. Participants were also given the option of completing the survey by phone. All participants were offered an honorarium worth $25.
Variables
For this study, the primary variable of interest was the proportion of participants who had recommended DNA banking for patients they suspected might have inherited cancer susceptibility. Specifically, participants were asked, “During the past 12 months, for about how many patients have you ordered DNA banking to allow future testing for inherited cancer susceptibility?” The surveys noted, “DNA banking refers to collecting and storing genetic material (usually a blood sample) that can be used in the future for clinical genetic testing or research.” This and other knowledge and behavior items were adapted from the National Cancer Institute's Physician Survey on Cancer Susceptibility Testing.11 This survey has successfully been used to assess knowledge and attitudes of a broad range of providers,9,10 though this is the first study to our knowledge that focuses on palliative care providers.
We also gathered information on a number of variables that might be associated with physicians' practices related to DNA banking. We assessed perceived self-efficacy for DNA banking by asking, “How qualified or unqualified do you consider yourself to recommend DNA banking because of inherited cancer susceptibility for your patients?” Responses ranged from “very well qualified” to “not qualified at all” on a 4-point scale with an additional option for “not sure.” Physicians were asked, “Based on your current knowledge, is DNA banking commercially available for your patients?” (yes, no, not sure). We assessed general knowledge of hereditary cancer through the following questions: “Suppose you have a female patient whose aunt or grandmother on her father's side carries a BRCA1 gene mutation for breast/ovarian cancer syndrome. In your opinion, could your patient also be a carrier of this mutation?” “In your opinion, what percentage of female breast cancer patients have a BRCA1 or BRCA2 mutation?” “In your opinion, what percentage of patients who carry a gene for hereditary nonpolyposis colorectal cancer (HNPCC) will actually go on to develop a colorectal cancer?”
We further assessed knowledge of hereditary cancer by asking participants whether commercially available tests were available for BRCA1/2, MLH1/MSH2, APC, and NF1. The same question was asked for two false scenarios: “MITF gene for hereditary sarcoma,” and “PAX3 gene for hereditary melanoma.”
A single multiple-response item asked participants, “What genetics resources or tools would be helpful for you in your clinical care of end-of-life cancer patients and their families?” Options were: Web-based risk assessment tool, genetic counselor on staff, educational brochures, telephone information hotline, continuing medical education opportunities, clinical decision-making algorithm, and other. Finally, we recorded the following relevant sociodemographic variables: race and ethnicity, year graduated from medical school, current age, and gender.
Study Size
Given the limited number of possible participants targeted for this study, we sought to enroll as many physicians nationally who met the inclusion criteria. In initial communication with ABHPM, we anticipated approximately 244 potential recruits; however, our ultimate list of potential participants was much lower (N = 133). We hoped for at least a 50% response rate. In a previous study of physicians, Wideroff et al9 achieved a 71% response rate for completing the National Cancer Institute's Physician Survey on Cancer Susceptibility Testing.
Statistical Analyses
We primarily conducted descriptive analyses, reporting the proportion of physicians who recommended DNA banking and their perceived qualifications to do so. For analyses, perceived qualification to recommend DNA banking was dichotomized as being at least “somewhat qualified.” A response of “not sure” was counted as a missing item.
In exploratory analyses, we conducted bivariate association tests of predictor variables (content knowledge of hereditary cancer, knowledge of the availability of genetic tests, knowledge of the availability of DNA banking, and sociodemographic variables). We created a dichotomous variable for content knowledge of hereditary cancer, reflecting participants who answered all content questions correctly (ie, women can inherit BRCA1 mutations from father's side, BRCA1/2 mutations account for < 10% of breast cancers, and the chance of colorectal cancer with HNPCC is at least 50%). For knowledge of the availability of various genetic tests, we created a score with +1 for correctly identifying BRCA1/2, MLH1/MSH2, APC, and NF1 as genes that are commercially available, and for correctly noting that MITF and PAX3 were not available commercially for cancer susceptibility testing. Participants answering “not sure” were counted as having incorrect responses, reflecting that they did not have adequate knowledge to be certain that testing is available or not. The summed range for this scale was 0 (no correct responses) to 6 (all correct responses). For nominal predictor variables, we used Fisher χ2 tests, and for continuous variables (knowledge of currently available tests, year of graduation from medical school, and current age),we used t tests. Predictors with significant bivariate associations were then included in logistic multiple regression models. All analyses were conducted using SAS 9.2 for Windows software.
Results
Data were collected from 49 (37%) of the possible 133 physicians. Almost all (96%) reported being of non-Hispanic white ethnicity. Thirty percent of participants were female. Mean age was 53.8 years (SD 7.8) with a range of 34 to 67. Average year graduating from medical school was 1980 (SD 7.8) and ranged from 1965 to 2001.
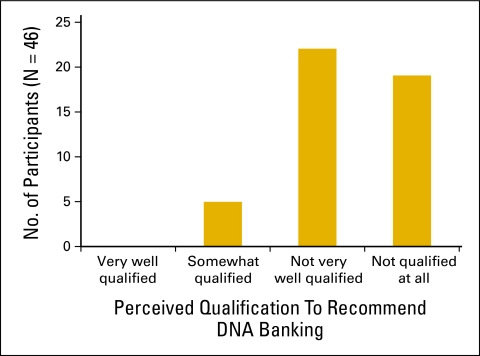
Thirty-nine (79.6%) participants reported assessing genetic risk for at least one patient. No participants reported banking patients' DNA during the previous 12 months. Five (10.6%) physicians knew of the commercial availability of DNA banking. No physicians reported being “very well qualified” to recommend DNA banking, and 19 (38.8%) reported being “not qualified at all” (Figure 1). Thirty-three (68.8%) physicians correctly responded that less than 10% of female patients with breast cancer have a BRCA1 or BRCA2 mutation, and the same number correctly noted that a BRCA1 mutation could be inherited from a woman's father. Fifteen (31.2%) correctly responded that the chance of colon cancer with HNPCC is between 50% and 100%. Nine (18.4%) participants responded correctly to all of the content knowledge questions. Forty-eight (98%) confirmed the availability of BRCA1/2 testing. Thirty-five (71%) reported knowing about genetic testing for HNPCC, and the same number confirmed the availability of testing for the APC gene associated with familial adenomatous polyposis. Of the six items asking about commercially available genetic tests, on average, participants gave correct responses for three (mean and median score). One participant gave correct responses for all six items.
Figure 1.
Palliative care oncologists' perceived qualifications to recommend DNA banking for patients suspected to have inherited cancer risk.
Because no physicians reported facilitating DNA banking, we assessed predictors of feeling at least “somewhat qualified” to recommend DNA banking, and we explored predictors of genetics assessment. No statistically significant associations were identified.
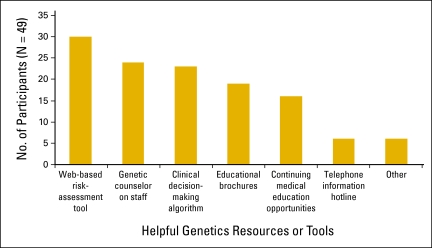
When asked to identify helpful resources, participants most commonly cited a Web-based risk assessment tool (61.2%), followed by a genetic counselor on staff (49.0%) and a clinical decision-making algorithm (46.9%) (Figure 2). There were no differences in resource preferences by perceived qualifications to recommend DNA banking.
Figure 2.
Physicians' preferences for genetics resources or tools to help with clinical care of end-of-life patients with cancer and their families.
Discussion
In the current genetic testing context, DNA banking might be the most practical genetics intervention for dying patients with cancer. The primary goal of our study was to assess current DNA banking practices among a group of physicians for whom this has high relevance, that is, those specializing in both oncology and palliative care. In our sample of 49 physicians, none reported offering DNA banking in the past year, suggesting a clear gap in service and highlighting potential opportunities in clinical care. Only 10% were aware of the availability of DNA banking. Perceived self-efficacy to order DNA banking was low, with approximately two-fifths of physicians feeling “not qualified at all.”
Knowledge about common genetic cancer susceptibility conditions did not predict self-efficacy for DNA banking. Physicians participating in the current study had similar knowledge about inherited breast cancer and HNPCC as oncologists in a previously conducted national survey by Wideroff et al.9 In that study, most oncologists (91%) were aware of commercially available BRCA1 and BRCA2 testing, whereas fewer knew about genetic testing for HNPCC (43%) and familial adenomatous polyposis (44%). Our sample showed a similar trend, with fewer knowing about the availability of genetic testing for colon cancer syndromes. Thus, although there is room for improvement, knowledge of specific genetic tests and cancer susceptibility conditions among participants in this study is comparable to national trends for oncologists and likely higher compared with that in other specialists. However, this relative increase in knowledge of genetic tests has not translated to greater knowledge or use of DNA banking.
Participants in this study provide insight about how these practice and knowledge gaps can be bridged. The most frequently proposed resource for DNA banking was a Web-based risk assessment tool. Electronic decision support tools will likely become increasingly important with greater use of electronic medical records. Oncologists are already familiar with many web-based resources like Adjuvant! Online (), the Breast Cancer Risk Assessment Tool (http://www.cancer.gov/bcrisktool), and nomograms for cancer recurrence (eg, http://www.mskcc.org/mskcc/html/15,938.cfm, http://www.mskcc.org/mskcc/html/10,088.cfm). Some online tools for cancer genetics referral exist [eg, MyGenerations (http://www.northsore.org/content.aspx?id=4411), Breast Cancer Genetics Referral Screening Tool (http://www.brcagenscreen.org)], and organizations such as the United States Preventive Services Task Force (http://www.uspreventiveservicestaskforce.org/uspstf/uspsbrgen.htm) and the National Comprehensive Cancer Network (http://www.nccn.org/professionals/physician_gls/f_guidelines.asp) have established Web-available guidelines for cancer genetic counseling referrals. However, there is little available currently to guide busy physicians' decisions about DNA banking. Our group has published some guidance through the End of Life/Palliative Education Resource Center (http://www.mcwedu/fastFact/ff_206.htm), but the efficacy of this resource has not been evaluated in clinical practice. A recent assessment of Web-based clinical genetics resources suggests time-efficient solutions are needed.12
Hiring a genetic counselor was the second-most-cited proposed resource. Recognition of the value of expert genetic counseling for identifying high-risk patients and facilitating genetic services is growing. Genetic counseling is recommended to accompany cancer susceptibility tests,1 and there is growing concern about inappropriate patient care and excessive cost when expert genetic services are not consulted.13 Physicians can search for nearby genetic counselors through the National Society of Genetic Counselors Web site (http://www.nsgc.org). Although genetic counselors are a logical resource for facilitating DNA banking in cancer care, we are not aware of an evidence base for this role, and this could represent a promising area for future investigation.
Study Limitations
This study has a number of limitations. Our study had a low response rate (37%) from an already limited sample population. Thus, the external validity of our results is questionable for palliative care oncologists. Furthermore, these specialists may not be representative of other specialists caring for dying patients who could benefit from genetics assessment. Roughly half of all patients with cancer now die with hospice services, so the insight from this study is relevant for hospice clinicians broadly. The small sample size limits the precision of findings within the study, and we also have low power to identify significant associations. However, considering that none of our participants reported ordering DNA banking, it seems clear that there is a need for educational intervention.
Our study is also limited by self-report of behaviors and knowledge. It is possible that participants did not remember ordering DNA banking when they actually had. However, considering only 10% reported even knowing of the availability of DNA banking services, this seems unlikely.
Implications for Oncology Practice
This study highlights a gap in oncology service and genetics knowledge. Although additional research is needed to verify our findings and to evaluate solutions, some actionable steps are immediately available (Figure 3). Oncologists cannot assume that end-of-life patients with cancer have been triaged for genetics assessment earlier in the disease process. In one study at a single institution, 21% of patients dying as a result of cancer had high genetic risk and none had previously been assessed.8 Therefore, clinicians caring for patients throughout the disease trajectory need to be mindful of a genetics component, and this includes palliative care clinicians. Furthermore, as palliative care providers are already accustomed to considering the familial social context, there is reason to extend the concept of medical necessity to family members as well. Genetic services will not likely be medically useful for the patient, but they could have life-and-death consequences for her family. Thus, family history assessment is important for end-of-life patients. Just as with all patients with cancer, oncologists need to ask about relatives encompassing three generations, noting types of cancers and ages of diagnosis. Talk with the patient about DNA banking and offer a genetics consult for patients with personal or family histories of early-onset cancer, multiple close relatives with the same types of cancer, or personal or family histories of individuals with multiple primary cancers, including those who have had negative genetic testing (eg, BRCA1/2 for hereditary breast and ovarian cancer, MLH1/MSH2 for Lynch syndrome) despite a strong family history and high suspicion. Even if family history is negative or unknown, a personal history of early-onset cancer, rare tumors, or cancers with high heritability (eg, medullary thyroid cancer, pheochromocytoma, male breast cancer, colon polyposis) could justify genetics assessment for the sake of relatives. An up-to-date listing of laboratories that offer clinical DNA banking services can be found at the GeneTests Web site (http://www.genetests.org). Certified genetic counselors can be found through the National Society of Genetic Counselors Web site (http://www.nsgc.org).
Figure 3.
Actionable steps for genetics assessment and offer of DNA banking for dying patients with cancer.
Summary
The present study provides new information about current clinical practices for DNA banking. Though the process is potentially valuable for surviving relatives, clinicians do not appear to be banking DNA for dying patients with cancer and generally do not feel qualified to do so. Future studies might investigate the potential for Web-based risk assessment tools or partnering with genetic counselors to ensure optimal legacies in end-of-life cancer care.
Acknowledgment
Supported by the Massey Cancer Center, Virginia Commonwealth University.
Authors' Disclosures of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
Author Contributions
Conception and design: John M. Quillin, Joann N. Bodurtha, Laura A. Siminoff, Thomas J. Smith
Provision of study materials or patients: Thomas J. Smith
Collection and assembly of data: John M. Quillin, Thomas J. Smith
Data analysis and interpretation: John M. Quillin, Thomas J. Smith
Manuscript writing: John M. Quillin, Laura A. Siminoff, Thomas J. Smith
Final approval of manuscript: John M. Quillin, Joann N. Bodurtha, Laura A. Siminoff, Thomas J. Smith
References
- 1.Robson ME, Storm CD, Weitzel J, et al. American Society of Clinical Oncology policy statement update: Genetic and genomic testing for cancer susceptibility. J Clin Oncol. 2010;28:893–901. doi: 10.1200/JCO.2009.27.0660. [DOI] [PubMed] [Google Scholar]
- 2.Stadler ZK, Vijai J, Thom P, et al. Genome-wide association studies of cancer predisposition. Hematol Oncol Clin North Am. 2010;24:973–996. doi: 10.1016/j.hoc.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 3.Wooster R, Weber BL. Breast and ovarian cancer. N Engl J Med. 2003;348:2339–2347. doi: 10.1056/NEJMra012284. [DOI] [PubMed] [Google Scholar]
- 4.Garber JE, Offit K. Hereditary cancer predisposition syndromes. J Clin Oncol. 2005;23:276–292. doi: 10.1200/JCO.2005.10.042. [DOI] [PubMed] [Google Scholar]
- 5.Quillin JM, Bodurtha JN, Smith TJ. Genetics assessment at the end of life: Suggestions for implementation in clinic and future research. J Palliat Med. 2008;11(3):451–458. doi: 10.1089/jpm.2007.0150. [DOI] [PubMed] [Google Scholar]
- 6.Skirton H, Frazier LQ, Calvin AO, et al. A legacy for the children–attitudes of older adults in the United Kingdom to genetic testing. J Clin Nurs. 2006;15:565–573. doi: 10.1111/j.1365-2702.2006.01372.x. [DOI] [PubMed] [Google Scholar]
- 7.Kirk J. The family history of cancer: A common concern in palliative care. Prog Palliat Care. 2004;12:59–65. [Google Scholar]
- 8.Quillin JM, Bodurtha JN, Siminoff LA, et al. Exploring hereditary cancer among dying cancer patients: A cross-sectional study of hereditary risk and perceived awareness of DNA testing and banking. J Genet Couns. 2010;19:497–525. doi: 10.1007/s10897-010-9308-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wideroff L, Vadaparampil ST, Greene MH, et al. Hereditary breast/ovarian and colorectal cancer genetics knowledge in a national sample of US physicians. J Med Genet. 2005;42:749–755. doi: 10.1136/jmg.2004.030296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Freedman AN, Wideroff L, Olson L, et al. US physicians' attitudes toward genetic testing for cancer susceptibility. Am J Med Genet A. 2003;120A:63–71. doi: 10.1002/ajmg.a.10192. [DOI] [PubMed] [Google Scholar]
- 11.National Cancer Institute. Physician Survey on Cancer Susceptibility Testing. http://riskfactor.cancer.gov/studies/physician/
- 12.Levy HP, LoPresti L, Seibert DC. Twenty questions in genetic medicine–An assessment of world wide web databases for genetics information at the point of care. Genet Med. 2008;10:659–667. doi: 10.1097/gim.0b013e318180639d. [DOI] [PubMed] [Google Scholar]
- 13.Brierley KL, Campfield D, Ducaine W, et al. Errors in delivery of cancer genetics services: Implications for practice. Conn Med. 2010;74:413–423. [PubMed] [Google Scholar]