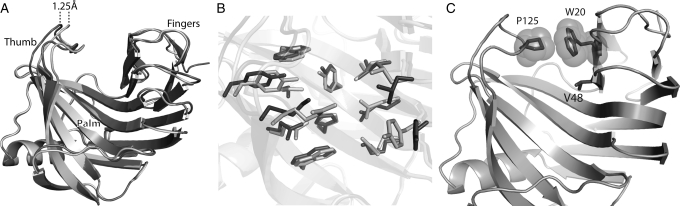
Fig. 3.
Backbone opening of the binding pocket and prediction of interface rotamer conformations between 1m4w_6-predicted model (light gray) and X-ray structure (dark gray). (A) Cartoon representation of the model and X-ray structure showing the 1.25 Å shift in the backbone configuration of the ‘thumb’ region. (B) Detailed comparison of the residues comprising the ligand interface. Most of the residue side chains are superimposable, while several are out of position due to the altered backbone conformation. Only two side-chain rotamers assume substantially different conformations from prediction. (C) Residues identified as directly responsible for binding pocket opening. W20–P125 (shown with VDW spheres) form a hydrophobic interaction between ‘thumb’ and ‘fingers’ at the top of the binding pocket, while V48 lies lower in the ‘palm’ of the protein.