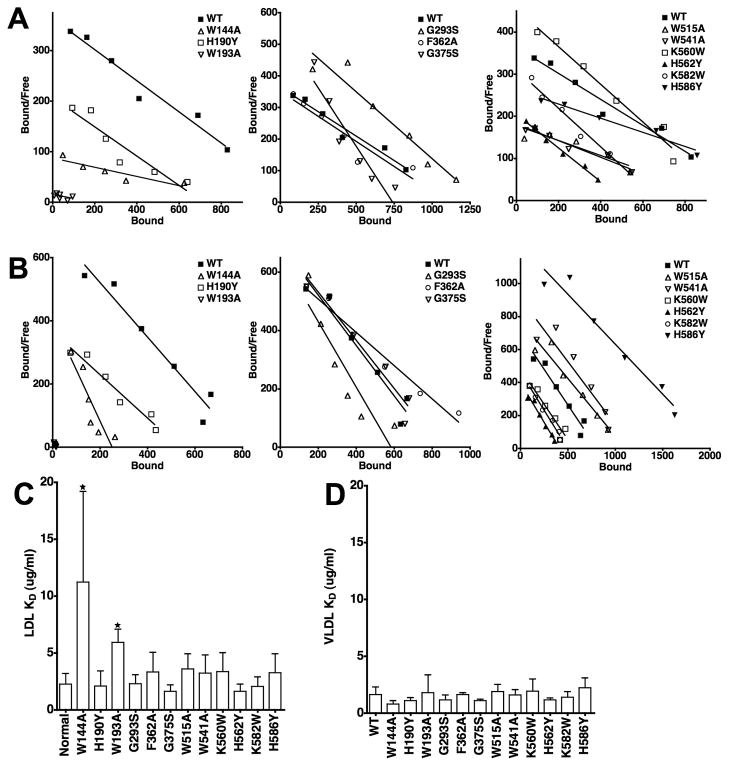
Fig. 6. Comparison of the ability of LDLR variants to bind LDL and β-VLDL.
Saturation binding assays were performed using 125I-LDL and 125I-β-VLDL for each of the variants. Representative data from three independent experiments is presented in Scatchard form for LDL (Panel A) and β-VLDL (Panel B) binding. The Scatchard plot data has been divided into three panels for clarity. WT data is the same in all three panels. Panels C and D show the average affinities from multiple determinations of LDL and β-VLDL binding. Error bars correspond to one standard deviation. W144A and W193A had significantly reduced affinity for LDL (*, p<0.05), but not β-VLDL. No other variant had a statistically significant difference in affinity for either lipoprotein as compared to cells expressing normal LDLR.