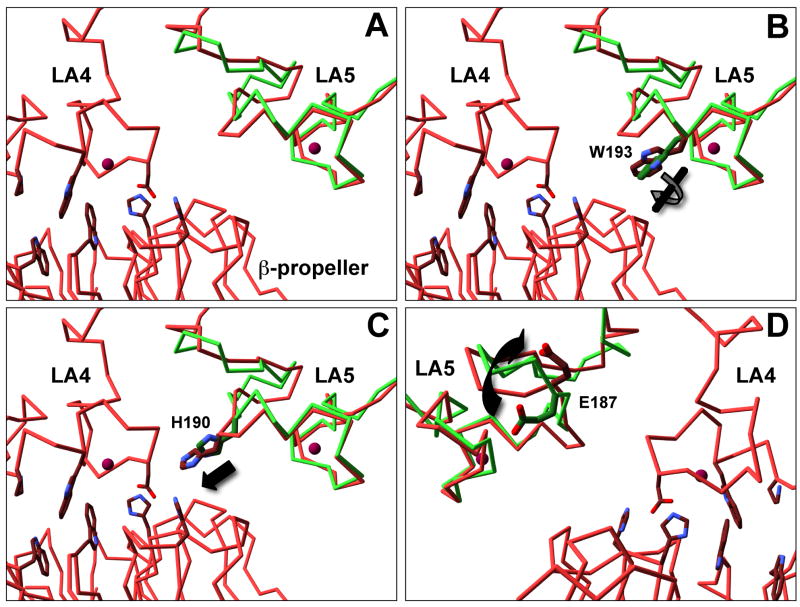
Fig. 7. LA5 has structural differences with and without the β-propeller bound.
Alpha carbons of LA5 alone at neutral pH (Green, PDB: 1AJJ) and LA5 as part of the ectodomain at acidic pH (Red, PDB 1N7D) were aligned using SwissPDB viewer (Panel A). Overall rms deviation over residues P175-C210 was 1.78 Å. The C-terminal half (W193-C210) aligned better than the N-terminal half (P175-W193) with an rms deviation of 1.38Å vs 1.97Å. Side chain differences of note include a twist in W193 (Panel B), a 1.5 Å movement of H190 (Panel C) and a flip in E187 (Panel D). The image in panel D is from the opposite side as that depicted in panels A–C.