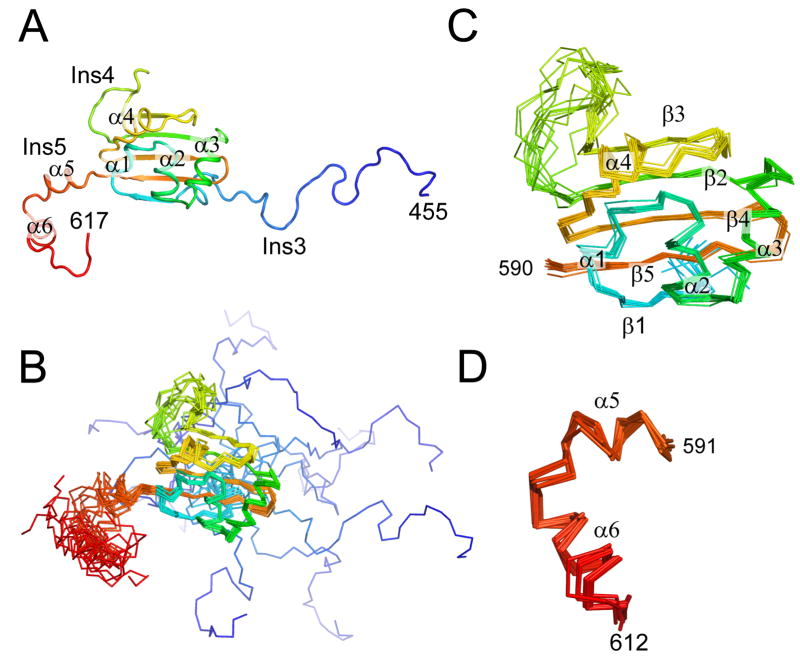
Figure 3.
NMR structure of the An mt TyrRS. (A) Cartoon diagram of a representative structure from the NMR ensemble. (B) Superposition of 12 structures of the C-terminal domain of the A. nidulans mt TyrRS that satisfy the NMR-derived constraints equally well. The positions of the β-strands and α-helices that form the core of the S4 fold are well-defined by the NMR data, while the locations of the termini and the loop of Ins4 between β2 and β3 are less certain. (C) Superposition of residues 490–590 from the 12 structures showing that the core of the S4 fold is well-defined by the NMR constraints. (D) Superposition of α5 and α6 of Ins5 from the 12 structures showing that the positions of these two helices are well-defined relative to each other, although their positions relative to the rest of the domain are not as well-defined (panel B). The color is ramped blue to red from the N- to C-terminus.