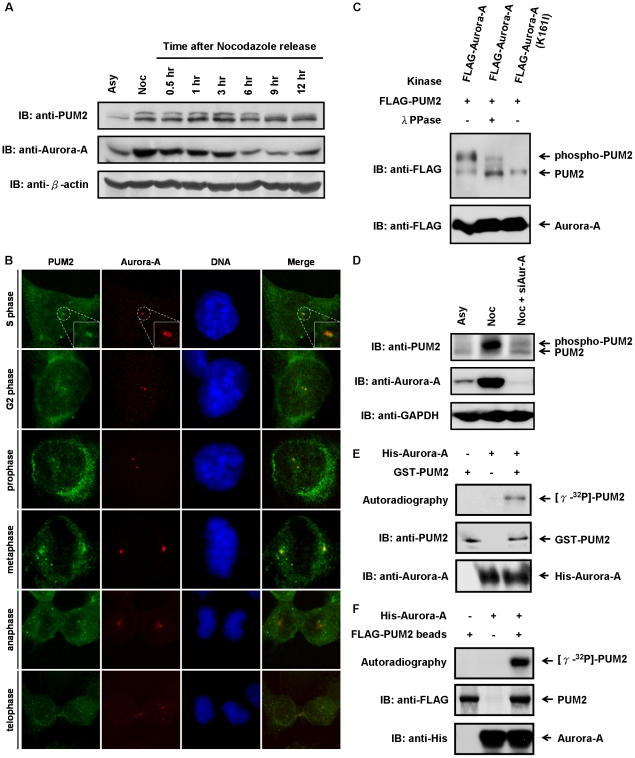
Figure 1. The cell cycle-regulated protein, PUM2, is a novel substrate of Aurora-A kinase.
(A) PUM2 exhibites remarkably variations both in protein quantity and phosphorylation state during exit from the G2/M block. HeLa cells were synchronized in the G2/M phase by treatment with nocodazole for 16 hrs and subsequently released into cell cycle progression by removal of the nocodazole. At the indicated time points, the cells were harvested and analyzed by immunoblotting. Asynchronously (Asy) growing cells were analyzed in parallel. (B) PUM2 was localized at the centrosomes from S phase to metaphase. The CL1–5 cells were fixed and probed with anti-PUM2 antibody (green) and anti-Aurora-A antibody (red), and the DNA was stained with DAPI (blue). The cells were visualized using confocal fluorescence microscopy. (C) PUM2 is a novel substrate for Aurora-A. HEK293T cells were transfected with FLAG-tagged PUM2 in combination with FLAG-tagged Aurora-A (the wild-type or kinase-inactive mutant). To confirm whether the gel mobility up-shift was derived from phosphorylated PUM2, the cell lysates were treated with and without λ protein phosphatase. (D) The M phase-specific electrophoretic mobility shift of PUM2 is abolished in Aurora-A-depleted cells. HeLa cells were transfected with Aurora-A specific siRNA (siAur-A) and synchronized in the G2/M phase by treatment with nocodazole for 16 hrs. The cells were harvested and analyzed by immunoblotting. (E, F) PUM2 is an in vitro substrate of Aurora-A. GST-tagged PUM2 (E) or FLAG-tagged PUM2 immunoprecipitated from cell lysates (F) was incubated, either alone or in combination with recombinant His-tagged Aurora-A, in the presence of [γ-32P]-ATP. The samples were electrophoresed using SDS-PAGE and transferred to a PVDF membrane. They were then either autoradiographed or immunoblotted.