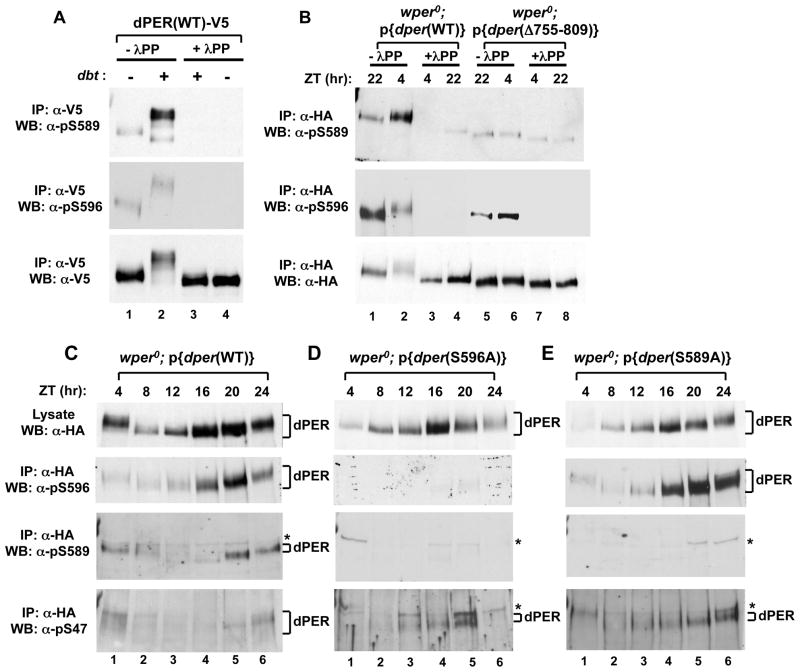
Figure 4. Phospho-specific antibodies reveal that DBT phosphorylates S589 but not S596, and that phosphorylation at S596 stimulates phosphorylation at S589 and S585, which delays phosphorylation at S47.
(A) Extracts were prepared from S2 cells co-transfected with a wild type (WT) version of dper (pAc-dper-V5-His) and pMT-dbt-V5-His (+) or an empty pMT plasmid, pMT-V5-His (−). Cells were treated with MG132 and cycloheximide 16 hours post-dbt induction and harvested 4 hrs later. dPER was immunoprecipitated (IP) with α-V5 beads and split into equal parts that were either treated in the absence (-λPP) or presence (+λPP) of λ-phosphatase. Recovered immune complexes were probed by western blotting (WB) in the presence of the indicated antibody (left of panel). (B) Head extracts were prepared from wper0;p{dper(WT)} or wper0;p{der(Δaa755-809)} flies collected at the indicated times (ZT; top of panels). dPER-HAHis-containing immune complexes were recovered using α-HA beads and split into two equal parts that were treated in the absence (-λPP) or presence (+λPP) of λ-phosphatase. Recovered immune complexes were probed by western blotting (WB) in the presence of the indicated antibody (left of panel). (C, D, E) Head extracts were prepared from wper0;p{dper(WT)}, wper0;p{dper(S589A)}, and wper0;p{dper(S596A)} flies collected at the indicated times (ZT). dPER-HAHis-containing immune complexes were recovered using α-HA beads, followed by western blotting (WB) in the presence of the indicated antibody (left of panel). Positions of non-specific signals from α-pS589 and α-pS47 antibodies are indicated by asterisks (*; right of panels). See Figs. S4 and S5 for supporting data.