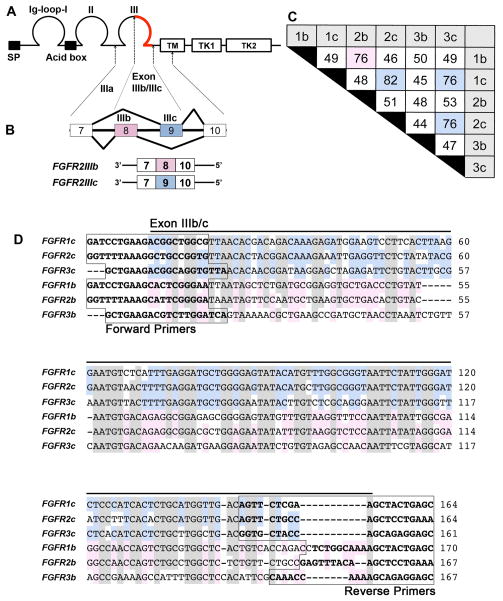
Figure 1. The FGF receptor isoforms generated by alternative splicing.
A, Diagram of the primary structure of FGF receptors showing a signal peptide (SP), three immunoglobulin-like (Ig) domains (labeled as Ig-loop-I, II, III), a stretch of seven conserved acidic amino acids between IgI and II (Acid box), a single transmembrane domain (TM), and two intracellular tyrosine kinase domains (TK1, 2). B, Alternative splicing of FGF receptors occurs in the IgIII domain, resulting in the corresponding ‘b’ or ‘c’ FGF receptor isoforms. As an example, FGFR2 is diagrammed to show that exons 8 and 9 are alternatively spliced to form the ‘b’ and ‘c’ isoforms. C, The nucleotide identity of the C-terminal half of the IgIII domain of each isoform is tabulated as percent identity, with an identity over 75% being highlighted in pink (isoform ‘b’) or blue (isoform ‘c’). The highest identity occurs between members of isoform ‘c’, with the highest identity of 82% occurring between FGFR1c and FGFR2c. D, The nucleotide sequence alignment of the C-terminal half of the IgIII domain of each isoform. High base pair identity occurs among all six isoforms (shown shaded in gray), with further identity within ‘c’ (blue) and ‘b’ (pink) subgroups. The boxed sequences indicate the location of primers for each isoform.