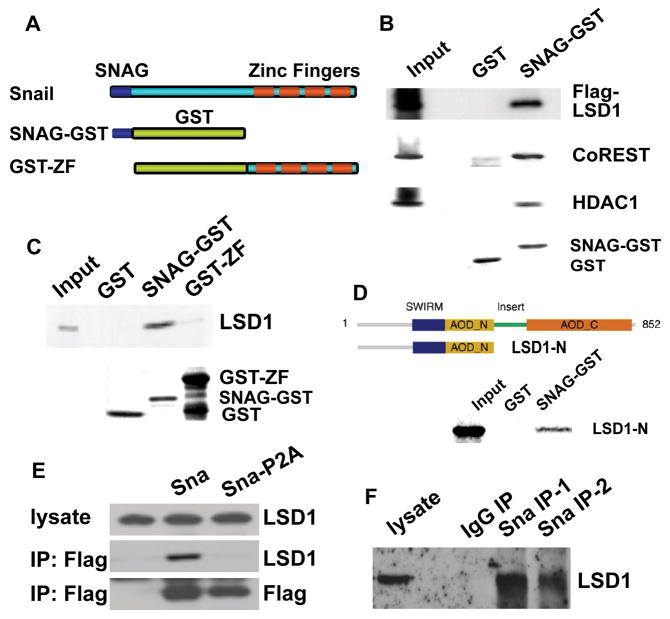
Figure 3. Snai1 physically interacts with LSD1 in vitro and in vivo.
(A) Schematic diagram of SNAG-GST and GST-ZF fusion proteins used in GST pull-down assays.
(B) The SNAG domain is sufficient for association with the LSD1 complex. Whole cell lysate prepared from HEK293 cells transfected with Flag-LSD1 was incubated with GST and the SNAG-GST fusion protein and followed by Western blot analysis using anti-Flag, anti-CoREST, and anti-HDAC1 antibodies. Coomassie staining shows the protein loading of GST and SNAG-GST.
(C) LSD1 directly interacts with the SNAG domain. 35S-labeled LSD1 protein was mixed with GST, SNAG-GST, or GST-ZF fusion proteins. Bound LSD1 was then detected by autoradiography after SDS/PAGE. Coomassie staining of GST proteins was shown.
(D) The amino-terminus of LSD1 interacts with SNAG.
(E) Exogenous Snai1 associates with endogenous LSD1 in intact cells. Co-immunoprecipitation of whole cell lysate from HEK293 cells overexpressing Flag-tagged wild-type Snai1 or Snai1-P2A mutant was performed with anti-Flag antibody. The presence of LSD1 in the precipitates was shown by Western blotting with an anti-LSD1 antibody. Anti-Flag Western blotting indicates expression of wild-type and mutant Snai1.
(F) Endogenous Snai1 and LSD1 form a complex in vivo. MDA-MB-231 cells were lysed and incubated with two anti-Snai1 antibodies (#1 fromSantaCruz; #2 from Cellsignaling) or control IgG, followed by Western blotting with the LSD1 antibody.