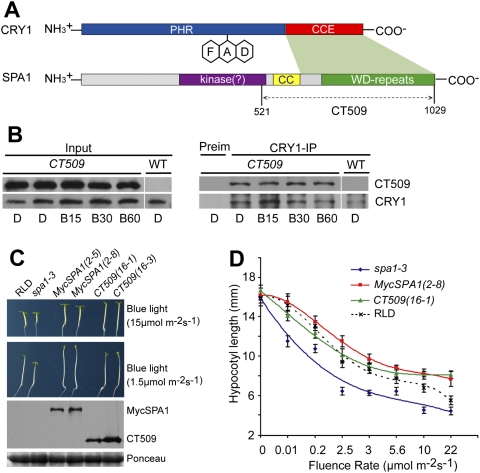
Figure 3.
The C-terminal domain of SPA1 interacts with CRY1 to affect the blue-light sensitivity of Arabidopsis seedlings. (A) A diagram depicting the domain organization of the SPA1 and CRY1 proteins. The different domains and regions involved in the CRY1–SPA1 interaction (green shade) are indicated. (B) A co-IP assay showing that CT509, which contains C-terminal 509 residues of SPA1, constitutively interacts with CRY1 in transgenic plants. The wild-type and MycCT509-expressing plants were grown in white light under a long-day photoperiod (16 h light /8 h dark) for 2 wk; plants were transferred to darkness for ∼18 h and exposed to blue light (20 μmol m−2 sec−1) for 15 min (B15), 30 min (B30), or 60 min (B60). Total extracts (Input), CRY1 (CRY1-IP), and control (Preim) immunoprecipitation products were fractionated by a SDS-PAGE gel, transferred to membranes, probed with the anti-Myc antibody (MycCT509), stripped, and reprobed with the anti-CRY1 antibody. (C) Five-day-old transgenic seedlings expressing MycSPA1 or MycCT509 (in the spa1-3 mutant background) and the controls were grown in continuous blue lights. Two independent lines of each genotype expressing the respective protein were tested, for which the respective levels of MycSPA1 or MycCT509 are shown in an immunoblot. (D) Hypocotyl lengths of the indicated genotypes grown in the dark or continuous blue light with different fluence rates for 5 d were measured and are shown. Standard deviations (n ≥ 20) are indicated.