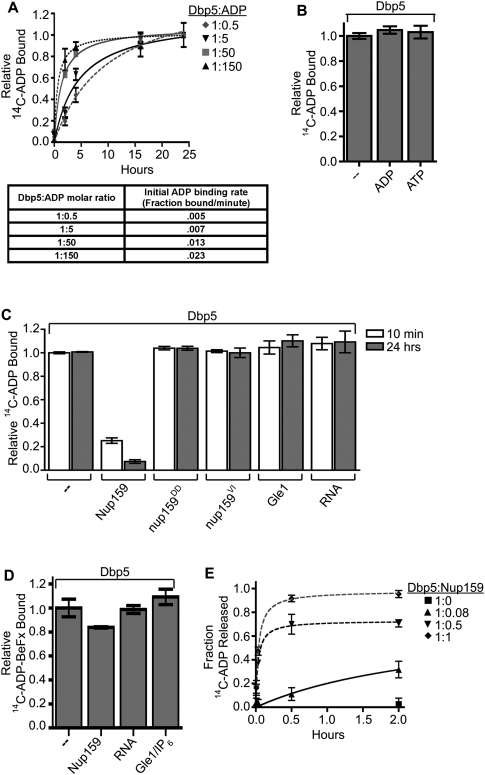
Figure 1.
Nup159 promotes Dbp5 release of ADP. (A) Equilibrium binding assays show an increase in initial rate of Dbp5–14C-ADP binding: Dbp5 (1 μM) was incubated with increasing concentrations of 14C-ADP (0.5–150 μM) over the course of 24 h. Samples were removed at several time points and used in filter-binding assays. Background integrated density signals of 14C-ADP at each concentration were subtracted from the Dbp5-bound ADP signal. As listed in the table, initial velocities of binding (fraction 14C-ADP bound per minute) were calculated during the linear phase of the binding curve at each Dbp5:14C-ADP ratio tested. (B) Dbp5 does not release bound ADP after equilibration: After 24-h equilibration of 1 μM Dbp5 and 5 μM 14C-ADP:MgCl2, excess unlabeled ATP:MgCl2 or ADP:MgCl2 (10 mM of each) was added, and filter-binding assays were conducted after an additional 24 h incubation. (C) Nup159-NTD does not promote release of the nonhydrolyzable ATP analog ADP-BeFx: After 26-h equilibration of 1 μM Dbp5 with 5 μM 14C-ADP-BeFx, either 1 μM Nup159NTD, 500 nM Gle1 with 200 nM IP6, or 2 μM poly(A)25 RNA was added, and filter-binding assays were performed at 24 h after addition. (D) Nup159-NTD specifically promotes Dbp5 release of bound ADP: After 24-h equilibration of 1 μM Dbp5 with 5 μM 14C-ADP:MgCl2, either 1 μM Nup159-NTD, 500 nM Gle1 with 200 nM IP6, or 2 μM RNA was added, and filter-binding assays were performed at 10 min and 24 h after addition. Two altered nup159 proteins (nup159DD and nup159VI) that do not bind Dbp5 in vitro were used as negative controls. (E) Increasing concentration of Nup159 promotes faster Dbp5 release of bound 14C-ADP. Dbp5 (1 μM) was incubated with 5 μM 14C-ADP for 24 h. Increasing concentrations of Nup159 were added and filter-binding assays were performed over the course of 2 h. All data points and bars represent at least three independent experiments with standard error bars.