Figure 5. Possible Organization of Liprin Scaffolds.
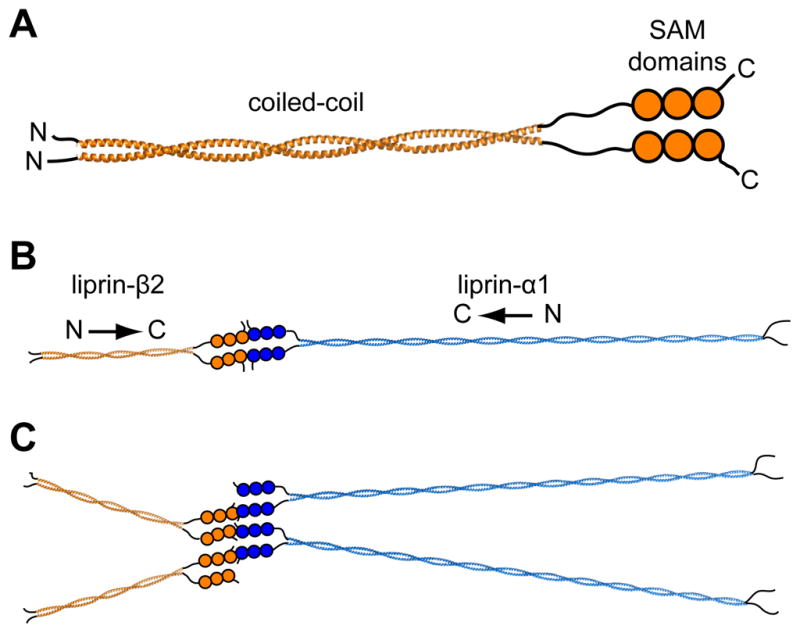
(A) A parallel homodimer model of the complete liprin-β2. The complete coiled-coil (~300 Å) was modeled using a combination of BEAMMOTIF and MAKECCSC.(42) The model is merely intended to convey the orientation and relative sizes of the coiled-coil domains. (B) A speculative scaffold organization is shown consisting of liprin-α1 and liprin-β2 molecules. For simplicity the entire liprin-α1 coiled-coil (~900 Å) was also assumed to form a two-stranded parallel structure which was constructed using MAKECCSC with no attempt to adjust for stutters, stammers, or gaps. The three tandem SAM domains of liprin-αs have been shown in other studies to interact with liprin-βs,(2) which we show here in a “closed dimer” model of α and β dimers. (C) An alternative “open scaffold” model is shown in which multiple α and β liprins stack indefinitely. In both “closed dimer” and “open scaffold” models the coiled-coil domains are predicted to radiate from the central SAM domain interactions.
