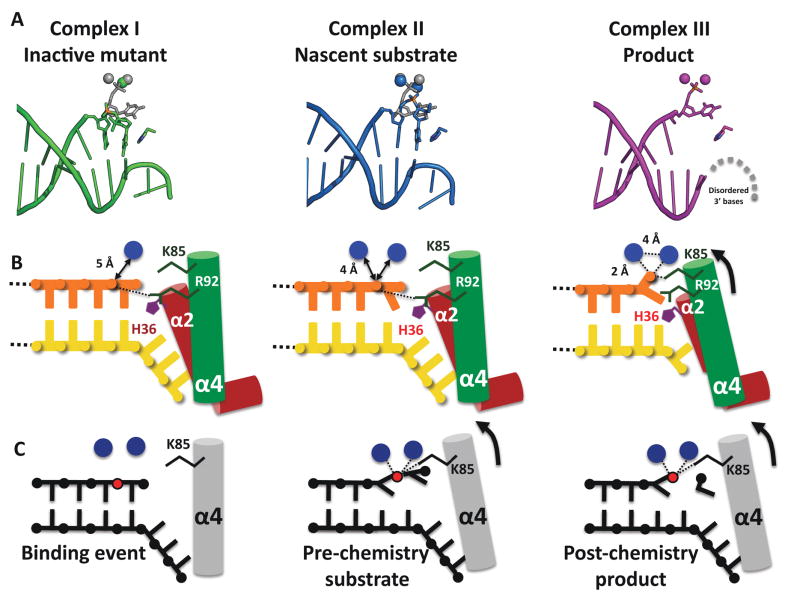
Figure 5. Assignment of structures to steps in the hExo1 reaction cycle.
(A) Comparison of active site in complex I (mutant), complex II (Ba2+ “nascent substrate”), and complex III (Mn2+ “product”) showing positions of DNA, metals, and H36.
(B) Schematic representation of some mechanistically relevant interactions. Complex I contains one Ca+ ion at some distance away from the phosphate in the scissile bond. Complex II contains two Ba2+ ions in the active site and exhibits 5′ basepair fraying. In both pre-chemistry structures the scissile 5′ phosphate interacts with R92. We propose that in a catalytically active metal complex, the scissile phosphate is bound by K85.
(C) Schematic representation of proposed hExo1 reaction cycle. After binding of the DNA and assembly of the metal sites (left), the scissile bond moves into the active site via phosphate coordination with two metal ions as well as K85 (center). Both the base poised for cleavage and the penultimate base are unpaired (frayed) from the duplex into the active site. Post-chemistry the 5′ phosphate of the product strand is coordinated by two metal ions (right).