Abstract
Lymphocytic or granulomatous hypophysitis is a rare entity with a difficult diagnosis. Our objective was to report a patient with non-tuberculous granulomatous hypophysitis. An HIV-negative 45-year old man with confusional state, subacute ophthalmoplegia, and clinical and laboratory findings of panhypopituitarism was seen in the emergency unit. A cranial MRI showed a sellar mass suggestive of hypophysitis. After an unsuccessful attempt with steroids and antituberculous drugs the patient died. Post-mortem histopathology revealed granulomatous lesions and restriction fragment length polymorphism analysis confirmed the presence of Mycobacterium gordonae’s DNA. In conclusion, we should consider granulomatous hypophysitis in the differential diagnosis of non-secreting hypophyseal tumors. The etiology of a pituitary granuloma by a non-tuberculous mycobacteria is best reached by histopathological techniques and molecular assays. The optimal therapy is yet to be established.
Key words: granuloma, hypophysis, non-tuberculous Mycobacteria, panhypopitituarism, pituitary gland.
Introduction
The pituitary region is susceptible to involvement by cystic, neoplastic, infectious and inflammatory processes.1 Granulomatous hypophysitis (GH) is an inflammatory disorder characterized by the formation of granulomas frequently associated with tuberculosis, sarcoidosis, syphilis, and lymphocytic adenohypophysitis. This entity usually presents with systemic symptoms such as high fever and hormonal disturbances.2
We describe a post-mortem case of granulomatous hypophysitis secondary to infection caused by Mycobacterium gordonae. To our knowledge, only two other cases of GH caused by non-tuberculous mycobacteria infection (Mycobacterium malmoense and Mycobacterium tokaiense) in non-compromised hosts have been reported to date.3,4
Case Report
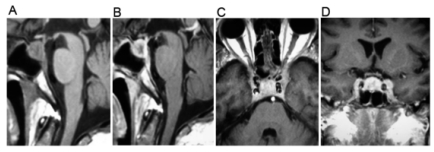
A 45-year-old man presented with a six-month history of weight loss, anorexia, vomiting, malaise and apathy. In the last month his condition worsened and headache, diplopia and left ptosis appeared. Neurological examination showed a person with slow mental processing, slow speech, affective flattening and left ophthalmoplegia (partial III cranial nerve palsy). No visual field disturbances, papilledema or meningeal signs were observed. General physical examination was unremarkable. Laboratory analyses only showed a low sodium blood level (114 mmol/L). A chest x-ray and a head CT scan were inconclusive and cerebrospinal fluid (CSF) was normal. After six days of hospitalization, fever, diarrhea and stupor appeared. A cranial MRI showed a sellar and parasellar heterogeneous mass, which in T1-weighted phase revealed a lesion with hypointense areas. In a T2-weighted phase this lesion was predominantly hyperintense with a hypointense center. After gadolinium administration, the lesion appeared heterogeneous with a parasellar extension toward the left cavernous sinus (Figure 1).
Figure 1.
A cranial magnetic resonance imaging showed an intrasellar mass. (A) A sagittal T1-weighted image revealed a sellar lesion with hypointense areas. (B) A gadolinium-enhanced sagittal image showed an enhancing lesion with a hypointense center. Axial (C) and coronal (D) images demonstrated parasellar extension toward the left cavernous sinus.
The measurement of plasma hypophysis hormones revealed a panhypopituitarism state. Based on the neuroimaging and hormonal findings, a presumptive diagnosis of hypophysitis was made. The patient was treated with steroid replacement, as well as with first- and second-line antituberculous drugs. Other laboratory studies were unremarkable, including serological tests for B and C hepatitis viruses, HIV, VDRL and Brucella, as well as erythrocyte sedimentation rate, C-reactive protein, antinuclear antibodies and rheumatoid factor.
Despite management, the patient died on day 11 of hospitilization. The autopsy showed an enlarged, fibrous and thickened pituitary gland. Microscopically, a granulomatous cellular reaction suggestive of mycobacterial disease was observed; however, Ziehl-Neelsen, modified Kinyoun and Auramine-Rodamine staining of the specimen did not identify any acid-fast bacteria (AFB). Molecular diagnostic testing for potential mycobacterial pathogens was then performed. Total DNA was extracted from the hypophysis specimen using a modification of the lysis method described by Sritharan and Barker.5 A PCR assay and restriction fragment length polymorphism (RFLP) analysis originally described by Roth et al. with oligonucleotide primers of the 16S-23S spacer was conducted.6 DNA amplification experiment included positive controls (for M. tuberculosis H37Rv, M. chelonae, and M. kansasii) and a blank as well as negative controls. The amplified products were digested separately with 2 IU of the restriction enzyme HaeIII and CfoI, (Sigma, St. Louis, MO, USA), according to the manufacturer’s recommendations. Fragment band sizes were estimated visually by comparison with appropriate controls (those positive for M. tuberculosis H37Rv, M. chelonae, and M. kansasii) in parallel with an extract of a whole hypophysis specimen and a 50 bp ladder (Fermentas, Hanover, USA). RFLP assay identified the genotypic characteristics of M. gordonae.
Discussion
Even after the HIV pandemics and the great use of immunosuppressive drugs, GH by non-tuberculous mycobacteria remains an uncommon disorder.7 The recognition of this entity in the differential diagnosis of abnormal sellar masses is important. In cases of GH it has been reported a disproportional degree of hormonal disturbances in relation to the size of the sellar mass.8 The most common manifestations are hypopituitarism, hyperprolactinemia, diabetes insipidus, visual field disturbances or aseptic meningitis. Nevertheless, none of these disorders is specific for this condition.1 Adequate diagnostic evaluation includes assessment of pituitary gland function, head magnetic resonance imaging and the investigation of the potential systemic disease. The trans-sphenoidal approach is the ideal method for diagnosis and local cure in masses suggestive of intrasellar granulomas, as it avoids cerebrospinal fluid contamination by infectious material.8 In our patient, the critical systemic and neurological condition made this surgical approach impossible.
The other2 reported cases of GH due to non-tuberculous mycobacteria with no history of immunosupression included a 32-year-old woman who presented with fever, headaches, nausea, vomiting and a six-month history of diabetes insipidus and amenorrhea.3 In the second case, a 36-year-old man presented with diabetes insipidus beginning three months prior, without other hormonal disturbances or any symptoms of mycobacterial meningitis. In both cases, Gd-MRI revealed an enhanced intrasellar mass of heterogeneous appearance with suprasellar extension and thickening of the pituitary stalk.4 In both patients, a mass lesion was completely removed and histological examination of the surgical specimen showed an epithelioid cell granuloma with caseous necrosis, but the acid-fast staining did not identify any AFB organisms. PCR and DNA sequencing to detect the non-tuberculous mycobacteria infection were used.
M. gordonae has been referred to as a tap water bacillus. It is also found in soil, whirlpools, and swimming pools. In healthy people it can be isolated from mucous membranes, urine, and gastric fluid. In the light of the widespread presence of this pathogen, infection with M. gordonae seems exclusively determined by host characteristics, and not by pathogen or exposure variables. M. gordonae has often been involved in pseudo-epidemics, where positive cultures were caused by contaminated tap water, fountains, ice machines, antimicrobial and laboratory solutions, aerosol devices and bronchoscopes.9–11 Despite this apparent vulnerability for our report, we believe this mycobacteria was responsible for this condition in view of all the clinical, radiological and histopathological test results.
The optimal therapy for patients with GH due to non-tuberculous mycobacteria is not defined in the literature. Although it is universally accepted that chemotherapy is essential for a successful treatment of intracranial granulomas, there is no consensus regarding the regimen of drugs or the duration of therapy.10 Antituberculous treatment must be opportunely initiated in patients who are thought to have GH since the mortality rate for mycobacterial central nervous system infection remains high.8
In conclusion, we should consider GH in the differential diagnosis of non-secreting hypophyseal tumors. The etiology of a pituitary granuloma by a non-tuberculous mycobacteria is best reached by histopathological techniques and molecular assays. The optimal therapy is yet to be established.
References
- 1.Lipscombe L, Asa SL, Ezzat S. Management of lesions of pituitary stalk and hipothalamus. Endocrinologist. 2003;13:38–51. [Google Scholar]
- 2.Honegger J, Fahlbusch R, Bornemann A, et al. Lymphocytic and granulomatous hypophysitis: Experience with nine cases. Neurosurgery. 1997;40:713–23. doi: 10.1097/00006123-199704000-00010. [DOI] [PubMed] [Google Scholar]
- 3.Florakis D, Kontogeorgos G, Anapliotou M, et al. Isolated pituitary granuloma by atypical Mycobacterium in a nonimmunosuppressed woman. Clin Endocrinol. 2002;56:123–6. doi: 10.1046/j.1365-2265.2002.01362.x. [DOI] [PubMed] [Google Scholar]
- 4.Kondo A, Mori K, Iwata J, et al. Caseus necrotic granuloma in the pituitary stalk due to nontuberculous mycobacteria (Mycobacterium Tokaiense) infection. Neur Med Chir. 2006;46:80–3. doi: 10.2176/nmc.46.80. [DOI] [PubMed] [Google Scholar]
- 5.Sritharan V, Barker RH. A simple method for diagnosis M. tuberculosis infection in clinical samples using PCR. Mol Cell Probes. 1991;5:385–95. doi: 10.1016/s0890-8508(06)80011-3. [DOI] [PubMed] [Google Scholar]
- 6.Roth A, Reischl U, Streubel A, et al. Novel diagnostic algorithm for identification of mycobacteria using genus-specific amplification of the 16S-23S rRNA gene spacer and restriction endonucleases. J Clin Microbiol. 2000;38:1094–104. doi: 10.1128/jcm.38.3.1094-1104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheung CC, Ezzat S, Smyth HS, Asa SL. The spectrum and significance of primary hypophysitis. J Clin Endocrinol Metab. 2006;86:1048–53. doi: 10.1210/jcem.86.3.7265. [DOI] [PubMed] [Google Scholar]
- 8.Sinha S, Singh A, Tatke M, Singh D. Hypophyseal tuberculoma: Direct radiosurgery is contraindicated for a lesion with a thickened pituitary stalk: Case report. Neurosurgery. 2000;46:735–8. doi: 10.1097/00006123-200003000-00041. [DOI] [PubMed] [Google Scholar]
- 9.Griffith DE, Aksamit T, Brown-Elliott BA, et al. An official ATS/IDSA statment: Diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Med. 2007;175:367–410. doi: 10.1164/rccm.200604-571ST. [DOI] [PubMed] [Google Scholar]
- 10.den Broeder AA, Vervoort G, van Assen S, et al. Disseminated Mycobacterium gordonae infection in a renal transplant recipient. Transpl Infect Dis. 2003;5:151–5. doi: 10.1034/j.1399-3062.2003.00016.x. [DOI] [PubMed] [Google Scholar]
- 11.Arnow PM, Bakir M, Thompson K, Bova JL. Endemic contamination of clinical specimens by Mycobacterium gordonae. Clin Infect Dis. 2000;31:472–6. doi: 10.1086/313940. [DOI] [PubMed] [Google Scholar]