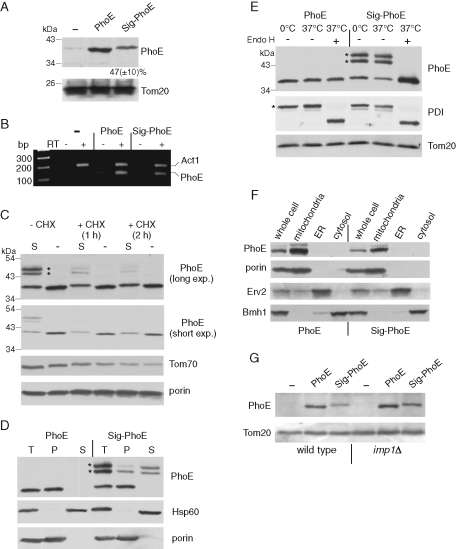
FIGURE 1:
Bacterial signal sequence interferes with assembly of PhoE into mitochondria. (A) Mitochondria isolated from yeast cells transformed with an empty plasmid (–) or with a plasmid encoding either mature PhoE (PhoE) or PhoE with its signal sequence (Sig-PhoE) were analyzed by SDS–PAGE and immunodecoration with antibodies against PhoE and Tom20 as a loading control. The intensity of the PhoE and Sig-PhoE bands in three independent experiments was quantified, and the amount of Sig-PhoE is expressed as mean (±SD)% of the level of PhoE. (B) mRNAs were isolated from the cells just described, and reverse transcriptase (RT) was added to the indicated samples. Then, PCR using primers complementary to PhoE or actin (as a control) was performed using the obtained DNA as template. The PCR-amplified DNA fragments were analyzed on a 2% agarose gel by staining with ethidium bromide. (C) Cells expressing either PhoE (indicated as “–”) or Sig-PhoE (indicated as “S“) were grown in the presence or absence of cycloheximide (CHX). Membrane fraction isolated from these cells was analyzed by SDS–PAGE and immunodecorated with antibodies against PhoE, Tom70, and porin. To better view the different intensities of the various PhoE forms, short and long exposures are presented. Modified forms of Sig-PhoE are indicated with an asterisk. (D) Membrane fractions isolated from cells expressing either PhoE or Sig-PhoE (total, T) were subjected to alkaline extraction and then centrifuged to discriminate between membrane proteins in the pellet (P) and soluble proteins in the supernatant (S). Proteins were analyzed by SDS–PAGE and immunodecorated with antibodies against PhoE, Hsp60 (soluble matrix protein), and porin (embedded in the outer membrane). Modified forms of Sig-PhoE are indicated with an asterisk. (E) Membrane fractions isolated from cells expressing either PhoE or Sig-PhoE were solubilized with a buffer containing 0.5% SDS and 40 mM dithiothreitol in the presence of protease inhibitor cocktail. The samples were then incubated for 1 h at 0°C (as control) or at 37°C in the presence or absence of Endoglycosidase Hf (Endo H). Proteins were analyzed by SDS–PAGE and immunodecorated with antibodies against PhoE, protein disulphide isomerase (PDI, a glycosylated ER protein), and Tom20 (a nonglycosylated mitochondrial protein). Glycosylated forms of Sig-PhoE and PDI are indicated with an asterisk. (F) Lysate of cells expressing either PhoE or Sig-PhoE and fractions corresponding to mitochondria, light microsomal fraction (ER), and cytosol were analyzed by SDS–PAGE and immunodecoration with antibodies against PhoE, the mitochondrial protein porin, the ER protein Erv2, and a marker protein for the cytosol (Bmh1). (G) An empty vector (–) or vector encoding for either PhoE or Sig-PhoE was transformed into either wild type or cells lacking a functional inner membrane peptidase (imp1Δ). Mitochondria isolated from these cells were analyzed by SDS–PAGE and immunodecoration with antibodies against PhoE and Tom20 as a loading control.