FIGURE 2:
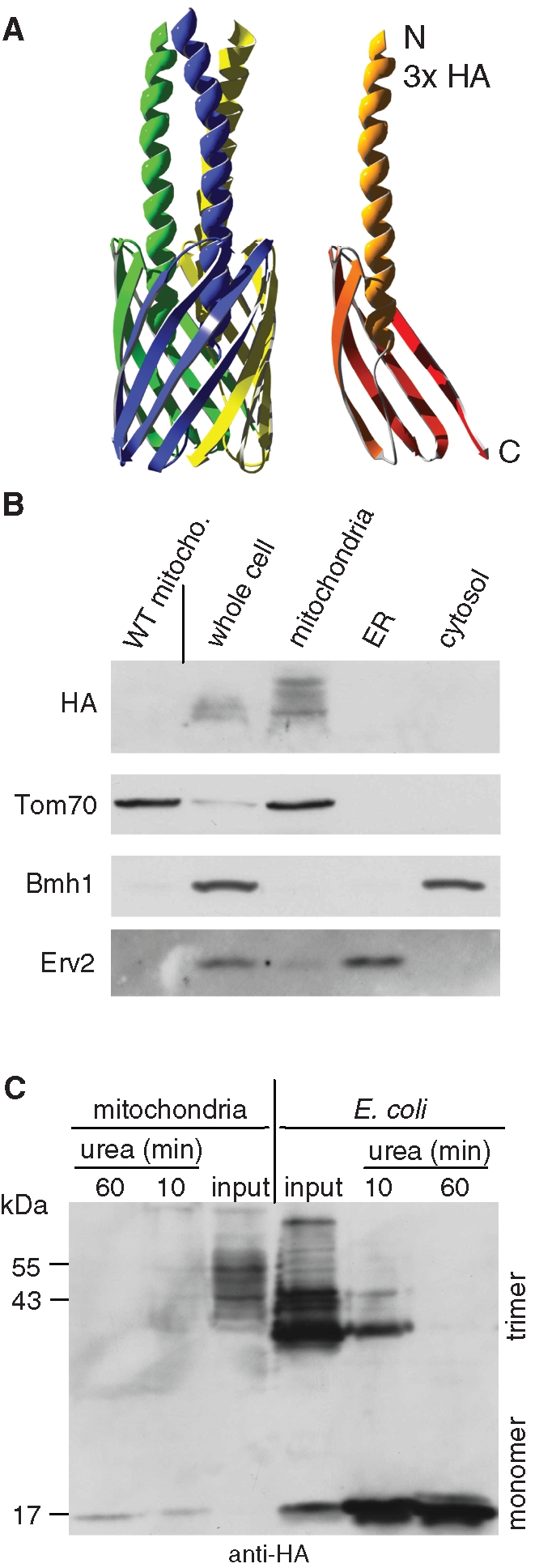
YadA-MA is assembled into mitochondria in a native trimeric conformation. (A) Atomic structure model of YadA-MA monomer with an HA-tag at its N-terminal (right) and trimeric form built from three monomers (left). Each YadA-MA monomer is composed of four β-strands that participate in the β-barrel structure and a linker domain (shown here as a helical structure). (B) YadA-MA is located in mitochondria. Lysate of cells expressing YadA-MA and fractions corresponding to mitochondria, ER, and cytosol were analyzed by SDS–PAGE and immunodecoration with antibodies against HA-tag, the mitochondrial protein Tom70, a marker protein for the cytosol (Bmh1), and the ER protein Erv2. Mitochondria isolated from untransformed wild-type cells were coanalyzed as a control. (C) Monomerization assay of YadA. Mitochondria isolated from yeast cells expressing YadA-MA and envelopes of E. coli–expressing YadA-MA were boiled for 5 min in Laemmli buffer without urea (input) or in Laemmli buffer containing 8 M urea for the indicated time periods. The samples were analyzed by SDS–PAGE and immunodecoration with HA-antibody. Molecular mass markers and the monomeric and trimeric forms of YadA are indicated (left and right, respectively).
