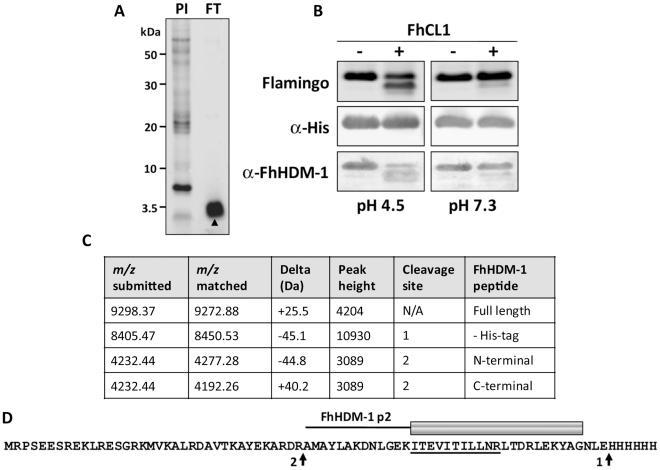
Figure 6. FhCL1 processes FhHDM-1 at low pH.
(A) Total secretory proteins from adult F. hepatica were concentrated from culture supernatants using a 3 kDa cut-off filter. 10 µg of the flow-through (FT) was analysed on a 4–12% Bi-Tris gel and stained with Flamingo fluorescent protein stain. The FT comprised a single prominent band (∼ 3.5 kDa) that was identified by LC-MS/MS as an FhHDM-1 fragment (high-scoring peptide ITEVITILLNR; m/z 642.93, underlined in D). N-terminal sequencing of this band was unsuccessful. (B) To investigate whether F. hepatica cathepsin L1 (FhCL1) can process FhHDM-1, 50 µg recombinant FhHDM-1 was incubated with 1 µg recombinant FhCL1 [35] in either 0.1 M sodium acetate (pH 4.5) or 0.1 M sodium phosphate (pH 7.3) each containing 1 mM EDTA and 1 mM DTT. Reactions were performed ± FhCL1 for 3 h at 37°C and stopped by the addition of E-64 (10 µM). Samples were analysed on 4–12% Bis-Tris gels and blots were probed with anti-His or anti-FhHDM-1 antibodies. (C) The pH 4.5 reaction in the presence of FhCL1 shown in (B) was analysed by MALDI-TOF MS. The major masses detected correspond to the full length recombinant FhHDM-1 ± the C-terminal His-tag (m/z 9272.88 and 8450.53 respectively) and two fragments (both m/z 4232.44) created by a single cleavage after Arg56 (native peptide numbering). (D) The putative FhHDM-1 cleavage sites are arrowed. Based on this, the synthetic peptide FhHDM-1 p2 was designed (shown as a cartoon above the primary sequence of recombinant FhHDM-1). Whilst trypsinising recombinant FhHDM-1 considerably reduced its interaction with LPS, boiling had no effect.