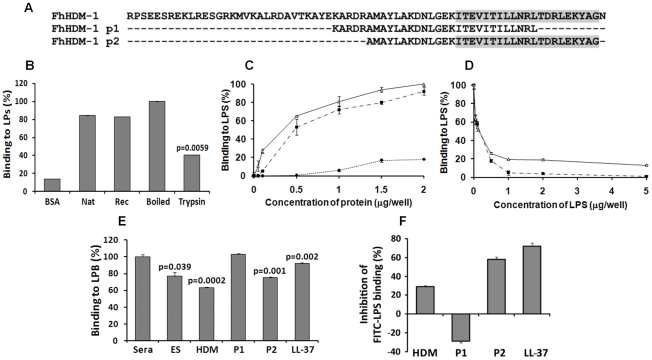
Figure 7. LPS neutralisation by FhHDM-1.
(A) Alignment of full-length FhHDM-1 with peptide 1 (FhHDM-1 p1) and peptide 2 (FhHDM-1 p2). The conserved C-terminal amphipathic helix is shaded in grey. (B) The ability of native and recombinant FhHDM-1 to bind LPS was investigated by incubating the proteins (2 µg/well) in an LPS-coated (100 ng/well) microtitre plate. Bound proteins were detected by ELISA using rabbit anti-FhHDM-1 as a primary antibody. BSA was used as a baseline control. Whilst trypsinising the recombinant FhHDM-1 significantly reduced the LPS interaction, boiling had no effect. (C) The ability of recombinant FhHDM-1 (Δ), FhHDM-1 p1 (•) or FhHDM-1 p2 (▪) to bind to LPS was investigated by incubating a range of concentrations of proteins (0.02–2 µg/well) in this assay. (D) FhHDM-1 or derived peptides (0.1 µg) were incubated in the presence of LPS (0.05-5 µg/well) and bound peptides measured as described above. Binding of peptides to the LPS-immobilised plates was expressed as a percentage of that measured for 2 µg (for panel C) or 0.1 µg (for panel D) of FhHDM-1. Data are the means ± SD from three separate experiments. (E) FhHDM-1 and FhHDM-1 p2 but not FhHDM-1 p1 reduced the interaction between LPS and LBP as effectively as LL-37. LPS-coated microtitre plates were incubated with 5 µg/well of F. hepatica ES, LL-37, FhHDM-1 or derived peptides for 1 h prior to the addition of 10% human sera in PBS. Interaction of LBP with LPS was measured by ELISA using an anti-LBP primary antibody and expressed as a percentage of that detected for 10% sera in the absence of added peptides. Data are the mean ± SD of three separate experiments. Statistical significance was calculated using the student t-test and represent a comparison to the binding of 10% sera to immobilised LPS. (F) Binding of FITC-conjugated LPS to RAW264.7 cells was inhibited by LL-37, FhHDM-1 and peptides. RAW264.7 cells (5×105cells/ml) were incubated with 100 ng/ml of FITC-conjugated LPS in the presence of FhHDM-1, FhHDM-1 p1, FhHDM-1 p2 and LL-37 (5 µg/ml) in RPMI 1640 containing 10% FBS for 20 min at 4°C. The binding of FITC-LPS was analysed by flow cytometry. Values represent percentage inhibition of FITC-LPS binding compared to cells in the absence of peptides. Data are the mean fluorescence ± SD of three independent experiments.