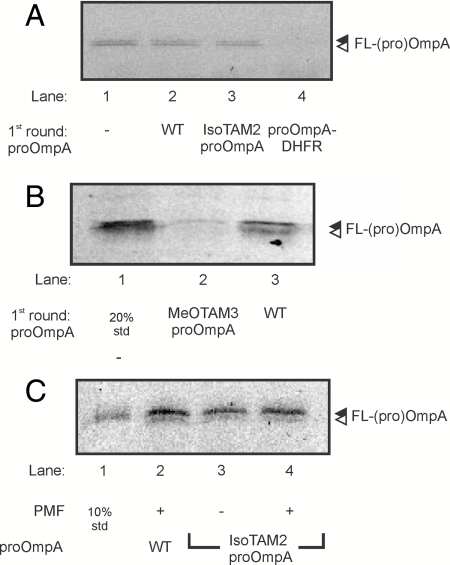
Fig. 4.
Translocation of MeOTAM3-proOmpA blocks the SecYEG pore. (A) IMVs containing overexpressed levels of SecYEG were used for a translocation reaction in the absence of proOmpA (lane 1), in the presence of wild-type proOmpA (lane 2), in the presence of IsoTAM2-proOmpA (lane 3), and in the presence of proOmpA-DHFR kept in its folded state by the addition of 1 mM NADPH and 50 μM methotrexate (lane 4). After 30 min at 37 °C the vesicles were recovered through a sucrose cushion and used for a second round of translocation with Fmal-labeled proOmpA. (B) Translocation reaction in the absence of proOmpA (lane 1), in the presence of wild-type proOmpA (lane 2), and in the presence of MeOTAM3-proOmpA (lane 3). After 30 min at 37 °C the vesicles were recovered through a sucrose cushion and used for a second round of translocation with Fmal-labeled proOmpA. All reactions were performed in the presence of a PMF. Samples were precipitated with TCA and protease protected material was analyzed by SDS-PAGE and in gel fluorescence. (C) Translocation of isoTAM2-proOmpA was performed with a limiting amount of IMVs in the presence and absence of a PMF. After 10 min at 37 °C, the reaction was stopped on ice and the PMF was dissipated in the reactions were translocation was performed in the presence of a PMF. Subsequently, the IMVs were isolated by centrifugation through a 0.8 M sucrose cushion and used in a second translocation reaction using Fmal-labeled proOmpA as substrate. After 10 min at 37 °C the reactions were stopped by the addition of proteinase K and incubated for 30 min on ice. Samples were precipitated with TCA and protease protected material was analyzed by SDS-PAGE (12% acrylamide) and in gel fluorescence. 10%: 10 percent of the Fmal-labeled proOmpA used in the translocation reaction.