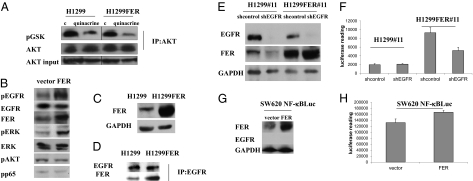
Fig. 4.
FER overexpression in the absence of EGF increases the phosphorylation of EGFR and ERK but not AKT; EGFR is important for FER-mediated activation of NF-κB. (A) FER overexpression does not activate the PI3K/AKT pathway. H1299 and H1299FER cells were treated with quinacrine (10 μM) for 24 h, cell lysates were immunoprecipitated with an antibody to AKT, and the products were analyzed in an in vitro kinase assay, with recombinant GSK as substrate, using an antibody to phosphorylated GSK. (B) FER overexpression induced the phosphorylation of EGFR and ERK. H1299 cells were transfected transiently with a control vector or a vector expressing FER. Cell lysates were analyzed by the Western method 48 h after transfection. (C and D) Interaction of FER with EGFR. (C) FER overexpression in H1299 FER cells was analyzed by the Western method with GAPDH as a loading control. (D) Extracts of H1299 and H1299FER cells were immunoprecipitated with an antibody to EGFR, and the products were analyzed by the Western method. (E and F) H1299#11 and H1299FER#11 cells were infected with vectors encoding a control shRNA or shRNAs against EGFR. (E) The levels of EGFR and FER were analyzed by the Western method with GAPDH as a loading control. (F) NF-κB–dependent luciferase activity was measured after infection. The lysates were normalized for protein concentration. The results of triplicate luciferase assays are shown as means ± SD. (G and H) SW620 cells with an integrated NF-κB–dependent luciferase reporter were infected with vectors encoding control or FER cDNAs. (G) The levels of EGFR and FER were examined by the Western method with GAPDH as a loading control. (H) NF-κB–dependent luciferase activity was measured after infection. The lysates were normalized for protein concentration. The results of triplicate luciferase assays are shown as means ± SD.